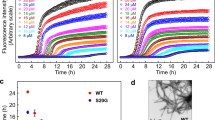
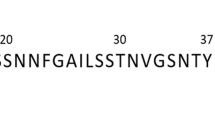Abstract
Understanding how small molecules interface with amyloid fibrils at the nanoscale is of importance for developing therapeutic treatments against amyloid-based diseases. Here, we show for the first time that human islet amyloid polypeptides (IAPP) in the fibrillar form are polymorphic, ambidextrous, and possess multiple periodicities. Upon interfacing with the small molecule epigallocatechin gallate (EGCG), IAPP aggregation was rendered off-pathway and assumed a form with soft and disordered clusters, while mature IAPP fibrils displayed kinks and branching but conserved the twisted fibril morphology. These nanoscale phenomena resulted from competitive interactions between EGCG and the IAPP amyloidogenic region, as well as end capping of the fibrils by the small molecule. This information is crucial in delineating IAPP toxicity implicated in type 2 diabetes and for developing new inhibitors against amyloidogenesis.

Similar content being viewed by others
References
Knowles, T. P. J.; Vendruscolo, M.; Dobson, C. M. The amyloid state and its association with protein misfolding diseases. Nat. Rev. Mol. Cell Biol. 2014, 15, 384–396.
Eisenberg, D.; Jucker, M. The amyloid state of proteins in human diseases. Cell 2012, 148, 1188–1203.
Sawaywa, M. R.; Sambashivan, S.; Nelson, R.; Ivanova, M. I.; Sievers, S. A.; Apostol, M. I.; Thompson, M. J.; Balbirnie, M.; Wiltzius, J. J.; McFarlane, H. T. et al. Atomic structures of amyloid cross-beta spines reveal varied steric zippers. Nature 2007, 447, 453–457.
Bieschke, J.; Herbst, M.; Wiglenda, T.; Friedrich, R. P.; Boeddrich, A.; Schiele, F.; Kleckers, D.; Lopez del Amo, J. M.; Grü ning, B. A.; Wang, Q. W. et al. Small-molecule conversion of toxic oligomers to nontoxic β-sheet-rich amyloid fibrils. Nat. Chem. Biol. 2012, 8, 93–101.
Bieschke, J.; Russ, J.; Friedrich, R. P.; Ehrnhoefer, D. E.; Wobst, H.; Neugebauer, K.; Wanker, E. E. EGCG remodels mature α-synuclein and amyloid-β fibrils and reduces cellular toxicity. Proc. Natl. Acad. Sci. USA 2010, 107, 7710–7715.
Sancini, G.; Dal Magro, R.; Ornaghi, F.; Balducci, C.; Forloni, G.; Gobbi, M.; Salmona, M.; Re, F. Pulmonary administration of functionalized nanoparticles significantly reduces beta-amyloid in the brain of an Alzheimer’s disease murine model. Nano Res. 2016, 9, 2190–2201.
Wang, N.; He, J. W.; Chang, A. K.; Wang, Y.; Xu, L. N.; Chong, X. Y.; Lu, X.; Sun, Y. H.; Xia, X. C.; Li, H. et al. (-)-Epigallocatechin-3-gallate inhibits fibrillogenesis of chicken cystatin. J. Agric. Food Chem. 2015, 63, 1347–1351.
Gurzov, E. N.; Wang, B.; Pilkington, E. H.; Chen, P. Y.; Kakinen, A.; Stanley, W. J.; Litwak, S. A.; Hanssen, E. G.; Davis, T. P.; Ding, F. et al. Inhibition of hIAPP amyloid aggregation and pancreatic β-cell toxicity by OH-terminated PAMAM dendrimer. Small 2016, 12, 1615–1626.
Nedumpully-Govindan, P.; Gurzov, E. N.; Chen, P. Y.; Pilkington, E. H.; Stanley, W. J.; Litwak, S. A.; Davis, T. P.; Ke, P. C.; Ding, F. Graphene oxide inhibits hIAPP amyloid fibrillation and toxicity in insulin-producing NIT-1 cells. Phys. Chem. Chem. Phys. 2016, 18, 94–100.
Mahmoudi, M.; Akhavan, O.; Ghavami, M.; Rezaee, F.; Ghiasi, S. M. A. Graphene oxide strongly inhibits amyloid beta fibrillation. Nanoscale 2012, 4, 7322–7325.
Li, M.; Zhao, A. D.; Dong, K.; Li, W.; Ren, J. S.; Qu, X. G. Chemically exfoliated WS2 nanosheets efficiently inhibit amyloid β-peptide aggregation and can be used for photothermal treatment of Alzheimer’s disease. Nano Res. 2015, 8, 3216–3227.
Nedumpully-Govindan, P.; Kakinen, A.; Pilkington, E. H.; Davis, T. P.; Ke, P. C.; Ding, F. Stabilizing off-pathway oligomers by polyphenol nanoassemblies for IAPP aggregation inhibition. Sci. Rep. 2016, 6, 19463.
Xu, C.; Shi, P.; Li, M.; Ren, J. S.; Qu, X. G. A cytotoxic amyloid oligomer self-triggered and NIR-enhanced amyloidosis therapeutic system. Nano Res. 2015, 8, 2431–2444.
Supattapone, S.; Nguyen, H. O. B.; Cohen, F. E.; Prusiner, S. B.; Scott, M. R. Elimination of prions by branched polyamines and implications for therapeutics. Proc. Natl. Acad. Sci. USA 1999, 96, 14529–14534.
Fischer, M.; Appelhan, D.; Schwar, S.; Klajner, B.; Bryszewsk, M.; Voi, B.; Rogers, M. Influence of surface functionality of poly(propylene imine) dendrimers on protease resistance and propagation of the scrapie prion protein. Biomacromolecules 2010, 11, 1314–1325.
Cegelski, L.; Pinkner, J. S.; Hammer, N. D.; Cusumano, C. K.; Hung, C. S.; Chorell, E.; Åberg, V.; Walker, J. N.; Seed, P. C.; Almqvist, F. et al. Small-molecule inhibitors target Escherichia coli amyloid biogenesis and biofilm formation. Nat. Chem. Biol. 2009, 5, 913–919.
Jones, O. G.; Mezzenga, R. Inhibiting, promoting, and preserving stability of functional protein fibrils. Soft Matter 2012, 8, 876–895.
Ehrnhoefer, D. E.; Bieschke, J.; Boeddrich, A.; Herbst, M.; Masino, L.; Lurz, R.; Engemann. S.; Pastore, A.; Wanker, E. E. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat. Struct. Mol. Biol. 2008, 15, 558–566.
Cao, P.; Raleigh, D. P. Analysis of the inhibition and remodeling of islet amyloid polypeptide amyloid fibers by flavanols. Biochemistry 2012, 51, 2670–2683.
Meng, F. L.; Abedini, A.; Plesner, A.; Verchere, C. B.; Raleigh, D. P. The flavanol (-)-epigallocatechin 3-gallate inhibits amyloid formation by islet amyloid polypeptide, disaggregates amyloid fibrils, and protects cultured cells against IAPP-induced toxicity. Biochemistry 2010, 49, 8127–8133.
Palhano, F. L.; Lee, J.; Grimster, N. P.; Kelly, J. W. Toward the molecular mechanism(s) by which EGCG treatment remodels mature amyloid fibrils. J. Am. Chem. Soc. 2013, 135, 7503–7510.
Engel, M. F. M.; vandenAkker, C. C.; Schleeger, M.; Velikov, K. P.; Koenderink, G. H.; Bonn, M. The polyphenol EGCG inhibits amyloid formation less efficiently at phospholipid interfaces than in bulk solution. J. Am. Chem. Soc. 2012, 134, 14781–14788.
vandenAkker, C. C.; Deckert-Gaudig, T.; Schleeger, M.; Velikov, K. P.; Deckert, V.; Bonn, M.; Koenderink, G. H. Nanoscale heterogeneity of the molecular structure of individual hIAPP amyloid fibrils revealed with tip-enhanced Raman spectroscopy. Small 2015, 11, 4131–4139.
Lara, C.; Gourdin-Bertin, S.; Adamcik, J.; Bolisetty, S.; Mezzenga, R. Self-assembly of ovalbumin into amyloid and non-amyloid fibrils. Biomacromolecules 2012, 13, 4213–4221.
Granqvist, C. G.; Buhrman, R. A. Ultrafine metal particles. J. Appl. Phys. 1976, 47, 2200–2219.
Usov, I.; Adamcik, J.; Mezzenga, R. Polymorphism in bovine serum albumin fibrils: Morphology and statistical analysis. Faraday Discuss. 2013, 166, 151–162.
Usov, I.; Mezzenga, R. FiberApp: An open-source software for tracking and analyzing polymers, filaments, biomacromolecules, and fibrous objects. Macromolecules 2015, 48, 1269–1280.
Adamcik, J.; Jung, J. M.; Flakowski, J.; De Los Rios, P.; Dietler, G.; Mezzenga, R. Understanding amyloid aggregation by statistical analysis of atomic force microscopy images. Nat. Nanotechnol. 2010, 5, 423–428.
Bolisetty, S.; Adamcik, J.; Mezzenga, R. Snapshots of fibrillation and aggregation kinetics in multistranded amyloid β-lactoglobulin fibrils. Soft Matter 2011, 7, 493–499.
Lara, C.; Adamcik, J.; Jordens, S.; Mezzenga, R. General self-assembly mechanism converting hydrolyzed globular proteins into giant multistranded amyloid ribbons. Biomacromolecules 2011, 12, 1868–1875.
Jeong, J. S.; Ansaloni, A.; Mezzenga, R.; Lashuel, H. A.; Dietler, G. Novel mechanistic insight into the molecular basis of amyloid polymorphism and secondary nucleation during amyloid formation. J. Mol. Biol. 2013, 425, 1765–1781.
Tycko, R. Molecular structure of amyloid fibrils: Insights from solid-state NMR. Q. Rev. Biophys. 2006, 39, 1–55.
Luca, S.; Yau, W. M.; Leapman, R.; Tycko, R. Peptide conformation and supramolecular organization in amylin fibrils: Constraints from solid-state NMR. Biochemistry 2007, 46, 13505–13522.
Reches, M.; Gazit, E. Casting metal nanowires within discrete self-assembled peptide nanotubes. Science 2003, 300, 625–627.
Knowles, T. P. J.; Waudby, C. A.; Devlin, G. L.; Cohen, S. I. C.; Aguzzi, A.; Vendruscolo, M.; Terentjev, E. M.; Welland, M. E.; Dobso. C. M. An analytical solution to the kinetics of breakable filament assembly. Science 2009, 326, 1533–1537.
Sang, S. M.; Lee, M.-J.; Hou, Z.; Ho, C. T.; Yang, C. S. Stability of tea polyphenol (-)-epigallocatechin-3-gallate and formation of dimers and epimers under common experimental conditions. J. Agric. Food Chem. 2005, 53, 9478–9484.
Knowles, T. P. J.; Buehler, M. J. Nanomechanics of functional and pathological amyloid materials. Nat. Nanotechnol. 2011, 6, 469–479.
Allen, M. P.; Tildesley, D. J. Computer Simulation of Liquids. Clarendon Press: Oxford, 1989.
Rapaport, D. C. The Art of Molecular Dynamics Simulation. 2nd ed.; Bar-Ilan University: Israel, 2004.
Ding, F.; Dokholyan, N. V. Emergence of protein fold families through rational design. PLoS Comput. Biol. 2006, 2, e85.
Yin, S. Y.; Biedermannova, L.; Vondrasek, J.; Dokholyan, N. V. MedusaScore: An accurate force field-based scoring function for virtual drug screening. J. Chem. Inf. Model. 2008, 48, 1656–1662.
Neria, E.; Fischer, S.; Karplus, M. Simulation of activation free energies in molecular systems. J. Chem. Phys. 1996, 105, 1902–1921.
Lazaridis, T.; Karplus, M. Effective energy functions for protein structure prediction. Curr. Opin. Struct. Biol. 2000, 10, 139–145.
Andersen, H. C. Molecular dynamics simulations at constant pressure and/or temperature. J. Chem. Phys. 1980, 72, 2384–2393.
Ding, F.; Dokholyan, N. V. Incorporating backbone flexibility in MedusaDock improves ligand-binding pose prediction in the CSAR2011 docking benchmark. J. Chem. Inf. Model. 2013, 53, 1871–1879.
Nedumpully-Govindan, P.; Jemec, D. B.; Ding, F. CSAR benchmark of flexible MedusaDock in affinity prediction and nativelike binding pose selection. J. Chem. Inf. Model. 2016, 56, 1042–1052.
Manning, G. S. Limiting Laws and counterion condensation in polyelectrolyte solutions I. Colligative properties. J. Chem. Phys. 1969, 51, 924–933.
Acknowledgements
This work was supported by ARC Project No. CE140100036 (T. P. D.), NSF CAREER grant CBET- 1553945 (F. D.), NIH 1R35GM119691 (F. D.), and an internal grant from Monash Institute of Pharmaceutical Sciences (P. C. K.).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Kakinen, A., Adamcik, J., Wang, B. et al. Nanoscale inhibition of polymorphic and ambidextrous IAPP amyloid aggregation with small molecules. Nano Res. 11, 3636–3647 (2018). https://doi.org/10.1007/s12274-017-1930-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-017-1930-7