Abstract
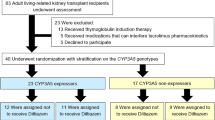
Chronic kidney disease (CKD) is a global health issue. Kidney failure patients may undergo a kidney transplantation (KTX) and prescribed an immunosuppressant medication i.e., tacrolimus. Tacrolimus’ efficacy and toxicity varies among patients. This study investigates the cost-utility of pharmacogenomics (PGx) guided tacrolimus treatment compared to the conventional approach in Austrian patients undergone KTX, participating in the PREPARE UPGx study. Treatment’s effectiveness was determined by mean survival, and utility values were based on a Visual Analog Scale score. Incremental Cost-Effectiveness Ratio was also calculated. PGx-guided treatment arm was found to be cost-effective, resulting in reduced cost (3902 euros less), 6% less hospitalization days and lower risk of adverse drug events compared to the control arm. The PGx-guided arm showed a mean 0.900 QALYs (95% CI: 0.862–0.936) versus 0.851 QALYs (95% CI: 0.814–0.885) in the other arm. In conclusion, PGx-guided tacrolimus treatment represents a cost-saving option in the Austrian healthcare setting.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Jones-Hughes T, Snowsill T, Haasova M, Coelho H, Crathorne L, Cooper C, et al. Immunosuppressive therapy for kidney transplantation in adults: a systematic review and economic model. NIHR J Libr. 2016;20:1–594.
Mudiayi D, Shojai S, Okpechi I, Christie EA, Wen K, Kamaleldin M, et al. Global estimates of capacity for kidney transplantation in world countries and regions. Transplantation. 2022;106:1113–22.
Chemello C, Aguilera M, Cañadas Garre M, Calleja MA. Pharmacogenetics and pharmacogenomics of chronic kidney disease comorbidities and kidney transplantation. In: Barh D et al., editors. Omics for personalized medicine. 1st ed. India: Springer eBooks; 2013. p.801–18.
Boenink R, Kramer A, Tuinhout RE, Savoye E, Åsberg A, Idrizi A, et al. Trends in kidney transplantation rate across Europe: study from the ERA Registry. Nephrol Dial Transpl. 2023;38:1528–39.
McEwan P, Dixon S, Baboolal K, Conway P, Currie CJ. Evaluation of the cost effectiveness of sirolimus versus tacrolimus for immunosuppression following renal transplantation in the UK. Pharmacoeconomics. 2006;24:67–79.
Glassock RJ, Warnock DG, Delanaye P. The global burden of chronic kidney disease: estimates, variability and pitfalls. Nat Rev Nephrol. 2017;13:104–14.
Rancic N, Dragojevic-Simic V, Vavic N, Kovacevic A, Segrt Z, Djordjevic N. Economic evaluation of pharmacogenetic tests in patients subjected to renal transplantation: a review of literature. Front Public Health. 2016;4:189–97.
Provenzani A, Santeusanio A, Mathis E, Notarbartolo M, Labbozzetta M, Poma P, et al. Pharmacogenetic considerations for optimizing tacrolimus dosing in liver and kidney transplant patients. World J Gastroenterol. 2013;19:9156–73.
Turolo S, Edefonti A, Syren ML, Montini G. Pharmacogenomics of old and new immunosuppressive drugs for precision medicine in kidney transplantation. J Clin Med. 2023;12:4454–70.
Adams SM, Crisamore KR, Empey PE. Clinical pharmacogenomics: applications in nephrology. Clin J Am Soc Nephrol. 2018;13:1561–71.
Nguyen TT, Pearson RA, Mohamed ME, Schladt DP, Berglund D, Rivers Z, et al. Pharmacogenomics in kidney transplant recipients and potential for integration into practice. J Clin Pharm Ther. 2020;45:1457–65.
Crowley LE, Mekki M, Chand S. Biomarkers and pharmacogenomics in kidney transplantation. Mol Diagn Ther. 2018;22:537–50.
Chen L, Prasad GVR. CYP3A5 polymorphisms in renal transplant recipients: influence on tacrolimus treatment. Pharmgenomics Pers Med. 2018;11:23–33.
Lamba J, Hebert JM, Schuetz EG, Klein TE, Altman RB. PharmGKB summary: very important pharmacogene information for CYP3A5. Pharmacogenet Genomics. 2012;22:555–8.
Swen JJ, van der Wouden CH, Manson LE, Abdullah-Koolmees H, Blagec K, Blagus T, et al. A 12-gene pharmacogenetic panel to prevent adverse drug reactions: an open-label, multicentre, controlled, cluster-randomised crossover implementation study. Lancet. 2023;401:347–56.
van der Wouden CH, Cambon-Thomsen A, Cecchin E, Cheung KC, Dávila-Fajardo CL, Deneer VH, et al. Implementing pharmacogenomics in Europe: design and implementation strategy of the ubiquitous pharmacogenomics consortium. Clin Pharm Ther. 2017;101:341–58.
Gordois AL, Toth PP, Quek RG, Proudfoot EM, Paoli CJ, Gandra SR. Productivity losses associated with cardiovascular disease: a systematic review. Expert Rev Pharmacoecon Outcomes Res. 2016;16:759–69.
Willan AR, Briggs A. Statistical analysis of cost‐effectiveness data. 1st ed. Canada: John Wiley & Sons Ltd; 2006.
General Hospital of Vienna, Clinical Insitute of Medical Analysis. Catalog of Parameters. 2023. https://www.akhwien.at/default.aspx?pid=3982.
Chung R, Howard K, Craig JC, Chapman JR, Turner R, Wong G. Economic evaluations in kidney transplantation: frequency, characteristics, and quality-a systematic review. Transplantation. 2014;97:1027–33.
Vannaprasaht S, Limwattananon C, Anutrakulchai S, Chan-On C. Effect of CYP3A5 genotype on hospitalization cost for kidney transplantation. Int J Clin Pharm. 2019;41:88–95.
Pasternak AL, Kidwell KM, Dempsey JM, Gersch CL, Pesch A, Sun Y, et al. Impact of CYP3A5 phenotype on tacrolimus concentrations after sublingual and oral administration in lung transplant. Pharmacogenomics. 2019;20:421–32.
Koufaki MI, Fragoulakis V, Díaz-Villamarín X, Karamperis K, Vozikis A, Swen JJ, et al. Economic evaluation of pharmacogenomic-guided antiplatelet treatment in Spanish patients suffering from acute coronary syndrome participating in the U-PGx PREPARE study. Hum Genomics. 2023;17:51–66.
Fragoulakis V, Roncato R, Bignucolo A, Patrinos GP, Toffoli G, Cecchin E, et al. Cost-utility analysis and cross-country comparison of pharmacogenomics-guided treatment in colorectal cancer patients participating in the U-PGx PREPARE study. Pharm Res. 2023;197:106949–58.
Skokou M, Karamperis K, Koufaki MI, Tsermpini EE, Pandi MT, Siamoglou S, et al. Clinical implementation of preemptive pharmacogenomics in psychiatry. eBioMedicine. 2024;101:105009–23.
Verbelen M, Weale ME, Lewis CM. Cost-effectiveness of pharmacogenetic-guided treatment: are we there yet? Pharmacogenomics J. 2017;17:395–402.
Funding
The project was funded by the European Commission Horizon 2020 Program via Grant Agreement 668353 (U-PGx; www.upgx.eu) to CM.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception, design and drafting of the paper. CJR and GSP performed data collection and documentation in eCRF and were part of the Austrian clinical team working on the PREPARE study. CM was one of the delegated persons, responsible for the design and the conduction of PREPARE project. VF and MIK reviewed and purified the study’s database. VF and MIK conducted data analysis. VF and MIK drafted the first and all subsequent versions of this paper. All authors reviewed and approved the final version of paper. VF and MIK are the guarantor for the overall content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study was approved by ethics committee of the Medical University of Vienna, Vienna, Austria.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fragoulakis, V., Koufaki, MI., Joefield-Roka, C. et al. Cost-utility analysis of pharmacogenomics-guided tacrolimus treatment in Austrian kidney transplant recipients participating in the U-PGx PREPARE study. Pharmacogenomics J 24, 10 (2024). https://doi.org/10.1038/s41397-024-00330-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41397-024-00330-5