Abstract
Purpose
Anthracycline-induced cardiotoxicity (AIC), whose major manifestation is diffuse myocardial fibrosis, is an important clinical problem in cancer therapy. Therefore, early identification and treatment are clinically important. This study aims to explore the feasibility of using 68 Ga-labelled fibroblast activation protein (FAP) inhibitor ([68 Ga]Ga-FAPI) positron emission tomography/computed tomography (PET/CT) for the early identification of the fibrotic process and guidance of antifibrosis therapy in AIC.
Methods
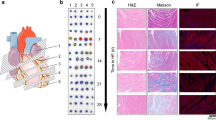
An AIC rat model was induced by the intravascular administration of doxorubicin (DOX) once per week for 1, 2, 3 and 6 weeks (2.5 mg/kg/injection, groups 1–4), whereas intravascular saline was administered to control rats. Experimental and control groups (n = 4) underwent [68 Ga]Ga-FAPI PET/CT following disease induction. Groups 5 and 6 received DOX injections for 3 and 6 weeks, treated with angiotensin-converting enzyme (ACE) inhibitor starting at 3 weeks, treated with enalapril (20 mg/kg, gastric gavage) daily and underwent echocardiography and [68 Ga]Ga-FAPI PET/CT at 3 weeks after treatment. Rat hearts were subjected to haematoxylin and eosin staining, FAP immunohistochemistry, Sirius red staining and Masson’s trichrome staining to investigate the pathological changes and deposition of collagen fibres. Rat blood was sampled weekly for the enzyme-linked immunosorbent assay of various markers of myocardial injury, such as plasma cardiac troponin I, B-type natriuretic peptide and angiotensin II.
Results
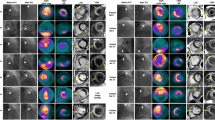
[68 Ga]Ga-FAPI-04 uptake by the heart was significantly higher in the cardiotoxicity group than in the control group at weeks 3 (SUVmax: 1.21 ± 0.23 vs 0.67 ± 0.01, P < 0.05) and 6 (SUVmax: 1.48 ± 0.28 vs 0.67 ± 0.08, P < 0.001), whereas left ventricle ejection fraction (LVEF) did not significantly differ between normal and AIC rats at week 3. FAP+ expression began to increase starting at week 3, before irreversible fibrotic changes were detected, until week 6. After 3 weeks of enalapril treatment, [68 Ga]Ga-FAPI-04 accumulation decreased in groups 5 and 6 (SUVmax decreased from 1.21 ± 0.23 to 0.77 ± 0.08 and 1.48 ± 0.28 to 1.09 ± 1.06, P < 0.05). Cardiac function was preserved (LVEF was 75.7% ± 7.38% in group 3 vs 74.5% ± 2.45% in group 5, P > 0.05) and improved (LVEF increased from 51.6% ± 9.03% in group 4 to 65.2% ± 4.27% in group 6, P < 0.05), and myocardial fibrosis attenuated (from 6.5% ± 1.2% in group 4 to 4.31% ± 0.37% in group 6, P < 0.01).
Conclusion
[68 Ga]Ga-FAPI PET/CT can be used for the early detection of active myocardial fibrosis in AIC and the evaluation of the efficacy of therapeutic interventions. Early treatment guided by [68 Ga]Ga-FAPI PET/CT may reduce anthracycline-induced myocardial injury and improve heart function.






Similar content being viewed by others
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Zeng F, Nijiati S, Liu Y, Yang Q, Liu X, Zhang Q, et al. Ferroptosis MRI for early detection of anticancer drug–induced acute cardiac/kidney injuries. Sci Adv. 2023;9. https://doi.org/10.1126/sciadv.add8539.
Zhang D, Xu Q, Wang N, Yang Y, Liu J, Yu G, et al. A complex micellar system co-delivering curcumin with doxorubicin against cardiotoxicity and tumor growth. Int J Nanomed. 2018;13:4549–61. https://doi.org/10.2147/ijn.S170067.
Force T, Kolaja KL. Cardiotoxicity of kinase inhibitors: the prediction and translation of preclinical models to clinical outcomes. Nat Rev Drug Discovery. 2011;10:111–26. https://doi.org/10.1038/nrd3252.
Chang H-M, Moudgil R, Scarabelli T, Okwuosa TM, Yeh ETH. Cardiovascular Complications of Cancer Therapy. J Am Coll Cardiol. 2017;70:2536–51. https://doi.org/10.1016/j.jacc.2017.09.1096.
Ewer MS, Ewer SM. Cardiotoxicity of anticancer treatments. Nat Rev Cardiol. 2015;12:547–58. https://doi.org/10.1038/nrcardio.2015.65.
Galán-Arriola C, Lobo M, Vílchez-Tschischke JP, López GJ, de Molina-Iracheta A, Pérez-Martínez C, et al. Serial magnetic resonance imaging to identify early stages of anthracycline-induced cardiotoxicity. J Am Coll Cardiol. 2019;73:779–91. https://doi.org/10.1016/j.jacc.2018.11.046.
Zhan H, Aizawa K, Sun J, Tomida S, Otsu K, Conway SJ, et al. Ataxia telangiectasia mutated in cardiac fibroblasts regulates doxorubicin-induced cardiotoxicity. Cardiovasc Res. 2016;110:85–95. https://doi.org/10.1093/cvr/cvw032.
Govender J, Loos B, Marais E, Engelbrecht AM. Mitochondrial catastrophe during doxorubicin-induced cardiotoxicity: a review of the protective role of melatonin. J Pineal Res. 2014;57:367–80. https://doi.org/10.1111/jpi.12176.
Farhad H, Staziaki PV, Addison D, Coelho-Filho OR, Shah RV, Mitchell RN, et al. Characterization of the changes in cardiac structure and function in mice treated with anthracyclines using serial cardiac magnetic resonance imaging. Circ: Cardiovasc Imaging. 2016:9. https://doi.org/10.1161/circimaging.115.003584.
Sobczuk P, Czerwińska M, Kleibert M, Cudnoch-Jędrzejewska A. Anthracycline-induced cardiotoxicity and renin-angiotensin-aldosterone system—from molecular mechanisms to therapeutic applications. Heart Fail Rev. 2020;27:295–319. https://doi.org/10.1007/s10741-020-09977-1.
Hiona A, Lee AS, Nagendran J, Xie X, Connolly AJ, Robbins RC, et al. Pretreatment with angiotensin-converting enzyme inhibitor improves doxorubicin-induced cardiomyopathy via preservation of mitochondrial function. J Thorac Cardiovasc Surg. 2011;142:396-403.e3. https://doi.org/10.1016/j.jtcvs.2010.07.097.
Abd El-Aziz MA, Othman AI, Amer M, El-Missiry MA. Potential protective role of angiotensin-converting enzyme inhibitors captopril and enalapril against adriamycin-induced acute cardiac and hepatic toxicity in rats. J Appl Toxicol. 2001;21:469–73. https://doi.org/10.1002/jat.782.
Zhao S. Letter to the editor: is it time for imaging to level with pathology? Int J Cardiovasc Imaging. 2020;36:2249–50. https://doi.org/10.1007/s10554-020-01936-z.
Lu M, Zhu L, Prasad SK, Zhao S. Magnetic resonance imaging mimicking pathology detects myocardial fibrosis: a door to hope for improving the whole course management. Science Bulletin. 2023;68:864–7. https://doi.org/10.1016/j.scib.2023.04.014.
Shaikh AY, Shih JA. Chemotherapy-induced cardiotoxicity. Curr Heart Fail Rep. 2012;9:117–27. https://doi.org/10.1007/s11897-012-0083-y.
Meléndez GC, Jordan JH, D’Agostino RB, Lesnefsky EJ, Hundley WG. Accelerated left ventricular interstitial collagen deposition after receiving doxorubicin in hypertension. J Am Coll Cardiol. 2018;72:1555–7. https://doi.org/10.1016/j.jacc.2018.07.028.
Ferreira de Souza T, Quinaglia A.C. Silva T, Osorio Costa F, Shah R, Neilan TG, Velloso L, et al. Anthracycline therapy is associated with cardiomyocyte atrophy and preclinical manifestations of heart disease. JACC Cardiovasc Imaging. 2018;11:1045–55. https://doi.org/10.1016/j.jcmg.2018.05.012.
Mayola MF, Thackeray JT. The potential of fibroblast activation protein-targeted imaging as a biomarker of cardiac remodeling and injury. Curr Cardiol Rep. 2023;25:515–23. https://doi.org/10.1007/s11886-023-01869-8.
Ivey MJ, Tallquist MD. Defining the cardiac fibroblast. Circ J. 2016;80:2269–76. https://doi.org/10.1253/circj.CJ-16-1003.
Kratochwil C, Flechsig P, Lindner T, Abderrahim L, Altmann A, Mier W, et al. 68Ga-FAPI PET/CT: tracer uptake in 28 different kinds of cancer. J Nucl Med. 2019;60:801–5. https://doi.org/10.2967/jnumed.119.227967.
Chen B-X, Xing H-Q, Gong J-N, Guo X-J, Xi X-Y, Yang Y-H, et al. Imaging of cardiac fibroblast activation in patients with chronic thromboembolic pulmonary hypertension. Eur J Nucl Med Mol Imaging. 2021;49:1211–22. https://doi.org/10.1007/s00259-021-05577-9.
Song W, Zhang X, He S, Gai Y, Qin C, Hu F, et al. (68)Ga-FAPI PET visualize heart failure: from mechanism to clinic. Eur J Nucl Med Mol Imaging. 2022. https://doi.org/10.1007/s00259-022-05994-4.
Varasteh Z, Mohanta S, Robu S, Braeuer M, Li Y, Omidvari N, et al. Molecular imaging of fibroblast activity after myocardial infarction using a (68)Ga-labeled fibroblast activation protein inhibitor, FAPI-04. J Nucl Med. 2019;60:1743–9. https://doi.org/10.2967/jnumed.119.226993.
Totzeck M, Siebermair J, Rassaf T, Rischpler C. Cardiac fibroblast activation detected by positron emission tomography/computed tomography as a possible sign of cardiotoxicity. Eur Heart J. 2020;41:1060. https://doi.org/10.1093/eurheartj/ehz736.
Xie B, Li L, Lin M, Nanna M, Su Y, Hua C, et al. 99mTc-HFAPi imaging identifies early myocardial fibrosis in the hypertensive heart. J Hypertens. 2023. https://doi.org/10.1097/hjh.0000000000003517.
Luo W, Zou X, Wang Y, Dong Z, Weng X, Pei Z, et al. Critical role of the cGAS-STING pathway in doxorubicin-induced cardiotoxicity. Circ Res. 2023:132. https://doi.org/10.1161/circresaha.122.321587.
Meng C, Fan L, Wang X, Wang Y, Li Y, Pang S, et al. Preparation and evaluation of animal models of cardiotoxicity in antineoplastic therapy. Oxid Med Cell Longev. 2022;2022:1–16. https://doi.org/10.1155/2022/3820591.
Fan Y, Liang L, Tang X, Zhu J, Mu L, Wang M, et al. Changes in the gut microbiota structure and function in rats with doxorubicin-induced heart failure. Front Cell Infect Microbiol. 2023:13. https://doi.org/10.3389/fcimb.2023.1135428.
Xiao F, Jiang H, Li Z, Jiang X, Chen S, Niu Y, et al. Reduced hepatic bradykinin degradation accounts for cold-induced BAT thermogenesis and WAT browning in male mice. Nat Commun. 2023:14. https://doi.org/10.1038/s41467-023-38141-0.
Leuschner F, Panizzi P, Chico-Calero I, Lee WW, Ueno T, Cortez-Retamozo V, et al. Angiotensin-converting enzyme inhibition prevents the release of monocytes from their splenic reservoir in mice with myocardial infarction. Circ Res. 2010;107:1364–73. https://doi.org/10.1161/circresaha.110.227454.
Wang G, Yang Q, Wu S, Xu X, Li X, Liang S, et al. Molecular imaging of fibroblast activity in pressure overload heart failure using [(68) Ga]Ga-FAPI-04 PET/CT. Eur J Nucl Med Mol Imaging. 2022. https://doi.org/10.1007/s00259-022-05984-6.
Sun F, Wang C, Feng H, Yu F, Zhang X, Zhang P, et al. Visualization of activated fibroblasts in heart failure with preserved ejection fraction with [18F]AlF-NOTA-FAPI-04 PET/CT imaging. Mol Pharm. 2023;20:2634–41. https://doi.org/10.1021/acs.molpharmaceut.3c00075.
Hu S, Gao Y, Gao R, Wang Y, Qu Y, Yang J, et al. The selective STING inhibitor H-151 preserves myocardial function and ameliorates cardiac fibrosis in murine myocardial infarction. Int Immunopharmacol. 2022;107: 108658. https://doi.org/10.1016/j.intimp.2022.108658.
Xu R, Ding Z, Li H, Shi J, Cheng L, Xu H, et al. Identification of early cardiac dysfunction and heterogeneity after pressure and volume overload in mice by high-frequency echocardiographic strain imaging. Front Cardiovasc Med. 2023:9. https://doi.org/10.3389/fcvm.2022.1071249.
Kourek C, Touloupaki M, Rempakos A, Loritis K, Tsougkos E, Paraskevaidis I, et al. Cardioprotective strategies from cardiotoxicity in cancer patients: a comprehensive review. J Cardiovasc Dev Dis. 2022:9. https://doi.org/10.3390/jcdd9080259.
Miller KD, Nogueira L, Devasia T, Mariotto AB, Yabroff KR, Jemal A, et al. Cancer treatment and survivorship statistics, 2022. CA: A Cancer J Clin. 2022:72:409–36. https://doi.org/10.3322/caac.21731.
Shan K. Anthracycline-induced cardiotoxicity. Ann Int Med. 1996:125. https://doi.org/10.7326/0003-4819-125-1-199607010-00008.
McGowan JV, Chung R, Maulik A, Piotrowska I, Walker JM, Yellon DM. Anthracycline chemotherapy and cardiotoxicity. Cardiovasc Drugs Ther. 2017;31:63–75. https://doi.org/10.1007/s10557-016-6711-0.
Cardinale D, Colombo A, Lamantia G, Colombo N, Civelli M, De Giacomi G, et al. Anthracycline-induced cardiomyopathy. J Am Coll Cardiol. 2010;55:213–20. https://doi.org/10.1016/j.jacc.2009.03.095.
Tan TC, Scherrer-Crosbie M. Assessing the cardiac toxicity of chemotherapeutic agents: role of echocardiography. Current Cardiovascular Imaging Reports. 2012;5:403–9. https://doi.org/10.1007/s12410-012-9163-3.
Armenian SH, Hudson MM, Mulder RL, Chen MH, Constine LS, Dwyer M, et al. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2015;16:e123–36. https://doi.org/10.1016/s1470-2045(14)70409-7.
Gonzalez A, Schelbert EB, Diez J, Butler J. Myocardial Interstitial Fibrosis in Heart Failure Biological and Translational Perspectives. J Am Coll Cardiol. 2018;71:1696–706. https://doi.org/10.1016/j.jacc.2018.02.021.
Packard RRS. Cardiac fibrosis in oncologic therapies. Curr Opin Physiol. 2022:29. https://doi.org/10.1016/j.cophys.2022.100575.
Carvalho FS, Burgeiro A, Garcia R, Moreno AJ, Carvalho RA, Oliveira PJ. Doxorubicin-induced cardiotoxicity: from bioenergetic failure and cell death to cardiomyopathy. Med Res Rev. 2014;34:106–35. https://doi.org/10.1002/med.21280.
Davis J, Molkentin JD. Myofibroblasts: trust your heart and let fate decide. J Mol Cell Cardiol. 2014;70:9–18. https://doi.org/10.1016/j.yjmcc.2013.10.019.
Teraoka K, Hirano M, Yamaguchi K, Yamashina A. Progressive cardiac dysfunction in adriamycin-induced cardiomyopathy rats. Eur J Heart Fail. 2000;2:373–8. https://doi.org/10.1016/s1388-9842(00)00111-2.
Cartas-Espinel I, Telechea-Fernández M, Manterola Delgado C, Ávila Barrera A, Saavedra Cuevas N, Riffo-Campos AL. Novel molecular biomarkers of cancer therapy-induced cardiotoxicity in adult population: a scoping review. ESC Heart Failure. 2022;9:1651–65. https://doi.org/10.1002/ehf2.13735.
Cardinale D, Colombo A, Sandri MT, Lamantia G, Colombo N, Civelli M, et al. Prevention of high-dose chemotherapy–induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation. 2006;114:2474–81. https://doi.org/10.1161/circulationaha.106.635144.
Burke RM, Lighthouse JK, Mickelsen DM, Small EM. Sacubitril/valsartan decreases cardiac fibrosis in left ventricle pressure overload by restoring PKG signaling in cardiac fibroblasts. Circ Heart Fail. 2019:12. https://doi.org/10.1161/circheartfailure.118.005565.
Cardinale D, Ciceri F, Latini R, Franzosi MG, Sandri MT, Civelli M, et al. Anthracycline-induced cardiotoxicity: a multicenter randomised trial comparing two strategies for guiding prevention with enalapril: the International CardioOncology Society-one trial. Eur J Cancer. 2018;94:126–37. https://doi.org/10.1016/j.ejca.2018.02.005.
Curigliano G, Cardinale D, Suter T, Plataniotis G, de Azambuja E, Sandri MT, et al. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann Oncol. 2012:23:vii155-vii66. https://doi.org/10.1093/annonc/mds293.
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–71. https://doi.org/10.1093/ehjci/jev014.
Funding
This research was supported by the National Key Research and Development Program of China (No. 2021YFF0501400 and 2021YFF0501404) and National Natural Science Foundation of China (No. 22277002 and 92059101).
Author information
Authors and Affiliations
Contributions
All authors’ material preparation and animal procedures were performed by Zhuxin Wei and Hongchuang Xu. Data collection and analysis were performed by Zhuxin Wei and Hongchuang Xu. Hongchuang Xu, Zhuxin Wei, Bixi Chen, Jiaxin Wang, Min-Fu Yang, Xing Yang and Shihua Zhao contributed to the study conception and design. Zhuxin Wei wrote the draft of the manuscript. Min-Fu Yang, Xing Yang and Shihua Zhao conceived the study and interpreted the results. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
This study was approved by the Animal Ethics Committee at Peking University Frist Hospital (no. J2023130).
Consent for publication
All authors of the current manuscript meet the specified criteria for authorship and agreed to publish this manuscript.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wei, Z., Xu, H., Chen, B. et al. Early detection of anthracycline-induced cardiotoxicity using [68 Ga]Ga-FAPI-04 imaging. Eur J Nucl Med Mol Imaging (2024). https://doi.org/10.1007/s00259-024-06673-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00259-024-06673-2