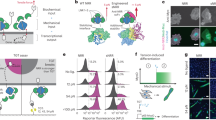
Abstract
Synthetic signaling receptors enable programmable cellular responses coupling with customized inputs. However, engineering a designer force-sensing receptor to rewire mechanotransduction remains largely unexplored. Herein, we introduce nongenetically engineered artificial mechanoreceptors (AMRs) capable of reprogramming non-mechanoresponsive receptor tyrosine kinases (RTKs) to sense user-defined force cues, enabling de novo-designed mechanotransduction. AMR is a modular DNA–protein chimera comprising a mechanosensing-and-transmitting DNA nanodevice grafted on natural RTKs via aptameric anchors. AMR senses intercellular tensile force via an allosteric DNA mechano-switch with tunable piconewton-sensitive force tolerance, actuating a force-triggered dynamic DNA assembly to manipulate RTK dimerization and activate intracellular signaling. By swapping the force-reception ligands, we demonstrate the AMR-mediated activation of c-Met, a representative RTK, in response to the cellular tensile forces mediated by cell-adhesion proteins (integrin, E-cadherin) or membrane protein endocytosis (CI-M6PR). Moreover, AMR also allows the reprogramming of FGFR1, another RTK, to customize mechanobiological function, for example, adhesion-mediated neural stem cell maintenance.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the article and its Supplementary Information. Source data are provided with this paper.
References
Allen, G. M. & Lim, W. A. Rethinking cancer targeting strategies in the era of smart cell therapeutics. Nat. Rev. Cancer 22, 693–702 (2022).
Sedlmayer, F., Aubel, D. & Fussenegger, M. Synthetic gene circuits for the detection, elimination and prevention of disease. Nat. Biomed. Eng. 2, 399–415 (2018).
Sánchez, M. F. & Tampé, R. Ligand-independent receptor clustering modulates transmembrane signaling: a new paradigm. Trends Biochem. Sci. 48, 156–171 (2023).
Manhas, J., Edelstein, H. I., Leonard, J. N. & Morsut, L. The evolution of synthetic receptor systems. Nat. Chem. Biol. 18, 244–255 (2022).
Iskratsch, T., Wolfenson, H. & Sheetz, M. P. Appreciating force and shape—the rise of mechanotransduction in cell biology. Nat. Rev. Mol. Cell Biol. 15, 825–833 (2014).
De Belly, H., Paluch, E. K. & Chalut, K. J. Interplay between mechanics and signalling in regulating cell fate. Nat. Rev. Mol. Cell Biol. 23, 465–480 (2022).
Chen, Y., Ju, L., Rushdi, M., Ge, C. & Zhu, C. Receptor-mediated cell mechanosensing. Mol. Biol. Cell 28, 3134–3155 (2017).
Liu, Y., Galior, K., Ma, V. P.-Y. & Salaita, K. Molecular tension probes for imaging forces at the cell surface. Acc. Chem. Res. 50, 2915–2924 (2017).
Morsut, L. et al. Engineering customized cell sensing and response behaviors using synthetic notch receptors. Cell 164, 780–791 (2016).
Stevens, A. J. et al. Programming multicellular assembly with synthetic cell adhesion molecules. Nature 614, 144–152 (2023).
Zhang, D. Y. & Seelig, G. Dynamic DNA nanotechnology using strand-displacement reactions. Nat. Chem. 3, 103–113 (2011).
Simmel, F. C., Yurke, B. & Singh, H. R. Principles and applications of nucleic acid strand displacement reactions. Chem. Rev. 119, 6326–6369 (2019).
Del Grosso, E., Franco, E., Prins, L. J. & Ricci, F. Dissipative DNA nanotechnology. Nat. Chem. 14, 600–613 (2022).
Lemmon, M. A. & Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 141, 1117–1134 (2010).
Trenker, R. & Jura, N. Receptor tyrosine kinase activation: from the ligand perspective. Cell Signal. 63, 174–185 (2020).
Müller, D. J. et al. Atomic force microscopy-based force spectroscopy and multiparametric imaging of biomolecular and cellular systems. Chem. Rev. 121, 11701–11725 (2021).
Kechagia, J. Z., Ivaska, J. & Roca-Cusachs, P. Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Biol. 20, 457–473 (2019).
Zhang, Y., Ge, C., Zhu, C. & Salaita, K. DNA-based digital tension probes reveal integrin forces during early cell adhesion. Nat. Commun. 5, 5167 (2014).
Bosco, A., Camunas-Soler, J. & Ritort, F. Elastic properties and secondary structure formation of single-stranded DNA at monovalent and divalent salt conditions. Nucleic Acids Res. 42, 2064–2074 (2014).
Ma, R. et al. DNA probes that store mechanical information reveal transient piconewton forces applied by T cells. Proc. Natl Acad. Sci. USA 116, 16949–16954 (2019).
Srinivas, N. et al. On the biophysics and kinetics of toehold-mediated DNA strand displacement. Nucleic Acids Res. 41, 10641–10658 (2013).
You, M. et al. DNA probes for monitoring dynamic and transient molecular encounters on live cell membranes. Nat. Nanotechnol. 12, 453–459 (2017).
Allingham, J. S., Smith, R. & Rayment, I. The structural basis of blebbistatin inhibition and specificity for myosin II. Nat. Struct. Mol. Biol. 12, 378–379 (2005).
Belardi, B., Son, S., Felce, J. H., Dustin, M. L. & Fletcher, D. A. Cell–cell interfaces as specialized compartments directing cell function. Nat. Rev. Mol. Cell Biol. 21, 750–764 (2020).
Zhao, B. et al. Visualizing intercellular tensile forces by DNA-based membrane molecular probes. J. Am. Chem. Soc. 139, 18182–18185 (2017).
Zhao, B. et al. Quantifying tensile forces at cell–cell junctions with a DNA-based fluorescent probe. Chem. Sci. 11, 8558–8566 (2020).
Uchikawa, E., Chen, Z., Xiao, G.-Y., Zhang, X. & Bai, X. Structural basis of the activation of c-MET receptor. Nat. Commun. 12, 4074 (2021).
Seveau, S. et al. Role of lipid rafts in E-cadherin–and HGF-R/Met–mediated entry of Listeria monocytogenes into host cells. J. Cell Biol. 166, 743–753 (2004).
Ueki, R., Atsuta, S., Ueki, A. & Sando, S. Nongenetic reprogramming of the ligand specificity of growth factor receptors by bispecific DNA aptamers. J. Am. Chem. Soc. 139, 6554–6557 (2017).
Kudo, T. et al. Live-cell measurements of kinase activity in single cells using translocation reporters. Nat. Protoc. 13, 155–169 (2018).
Gille, H., Sharrocks, A. D. & Shaw, P. E. Phosphorylation of transcription factor p62TCF by MAP kinase stimulates ternary complex formation at c-fos promoter. Nature 358, 414–417 (1992).
Liu, Y., Yehl, K., Narui, Y. & Salaita, K. Tension sensing nanoparticles for mechano-imaging at the living/nonliving interface. J. Am. Chem. Soc. 135, 5320–5323 (2013).
Lecuit, T. & Yap, A. S. E-cadherin junctions as active mechanical integrators in tissue dynamics. Nat. Cell Biol. 17, 533–539 (2015).
Banik, S. M. et al. Lysosome-targeting chimaeras for degradation of extracellular proteins. Nature 584, 291–297 (2020).
Gordon, W. R. et al. Mechanical allostery: evidence for a force requirement in the proteolytic activation of notch. Dev. Cell 33, 729–736 (2015).
von Kleist, L. et al. Role of the clathrin terminal domain in regulating coated pit dynamics revealed by small molecule inhibition. Cell 146, 471–484 (2011).
Diamandis, P. et al. Chemical genetics reveals a complex functional ground state of neural stem cells. Nat. Chem. Biol. 3, 268–273 (2007).
Karpowicz, P. et al. E-Cadherin regulates neural stem cell self-renewal. J. Neurosci. 29, 3885–3896 (2009).
Ferreira, T. A. et al. Neuronal morphometry directly from bitmap images. Nat. Methods 11, 982–984 (2014).
Sloas, D. C., Tran, J. C., Marzilli, A. M. & Ngo, J. T. Tension-tuned receptors for synthetic mechanotransduction and intercellular force detection. Nat. Biotechnol. 41, 1287–1295 (2023).
Wang, X. & Ha, T. Defining single molecular forces required to activate integrin and notch signaling. Science 340, 991–994 (2013).
Ma, V. P.-Y. & Salaita, K. DNA nanotechnology as an emerging tool to study mechanotransduction in living systems. Small 15, 1900961 (2019).
Li, H. et al. A reversible shearing DNA probe for visualizing mechanically strong receptors in living cells. Nat. Cell Biol. 23, 642–651 (2021).
Zhang, Z. et al. Programmable integrin and N-cadherin adhesive interactions modulate mechanosensing of mesenchymal stem cells by cofilin phosphorylation. Nat. Commun. 13, 6854 (2022).
Shaw, A. et al. Spatial control of membrane receptor function using ligand nanocalipers. Nat. Methods 11, 841–846 (2014).
Li, J., Green, A. A., Yan, H. & Fan, C. Engineering nucleic acid structures for programmable molecular circuitry and intracellular biocomputation. Nat. Chem. 9, 1056–1067 (2017).
Mills, A. et al. A modular spring-loaded actuator for mechanical activation of membrane proteins. Nat. Commun. 13, 3182 (2022).
Saminathan, A. et al. A DNA-based voltmeter for organelles. Nat. Nanotechnol. 16, 96–103 (2021).
Woodside, M. T. et al. Nanomechanical measurements of the sequence-dependent folding landscapes of single nucleic acid hairpins. Proc. Natl Acad. Sci. USA 103, 6190–6195 (2006).
Prezhdo, O. V. & Pereverzev, Y. V. Theoretical aspects of the biological catch bond. Acc. Chem. Res. 42, 693–703 (2009).
Wang, X. et al. Constructing modular and universal single molecule tension sensor using protein G to study mechano-sensitive receptors. Sci. Rep. 6, 21584 (2016).
Ovesný, M., Křížek, P., Borkovec, J., Švindrych, Z. & Hagen, G. M. ThunderSTORM: a comprehensive ImageJ plug-in for PALM and STORM data analysis and super-resolution imaging. Bioinformatics 30, 2389–2390 (2014).
Ricci, F., Vallée-Bélisle, A., Porchetta, A. & Plaxco, K. W. Rational design of allosteric inhibitors and activators using the population-shift model: in vitro validation and application to an artificial biosensor. J. Am. Chem. Soc. 134, 15177–15180 (2012).
Bai, R. et al. Paper-based 3D scaffold for multiplexed single cell secretomic analysis. Anal. Chem. 90, 5825–5832 (2018).
Acknowledgements
We thank Y. Lu and Y. H. Ji (Dalian Institute of Chemical Physics, Chinese Academy of Sciences) for their technical support in the preparation of high-density microwell array chips. We thank H. D. Wang and J. Gao (Changchun Institute of Applied Chemistry, Chinese Academy of Sciences) for providing technical assistance on dSTORM imaging and data analysis. We thank N. Cai (Cellway Biotechnology Co., Ltd) for technical discussions in cell culture of NSCs. This research was supported by the National Key Research and Development Program of China (grant nos. 2020YFA0907500 and 2021YFA0910100 to Z.N.) and the National Natural Science Foundation of China (grant nos. 22034002 and 92253304 to Z.N., 22177030 to H.h.W.).
Author information
Authors and Affiliations
Contributions
Z.N. conceived and designed the project. S.Y., H.h.W. and Z.N. designed, and S.Y. performed the experiments. M.L., S.L., D.T. and X.Z. provided technical assistance for AFM-related experiments. S.Y., M.W., K.C., H.h.W. and Z.N. analyzed the data. Z.N., H.h.W, and S.Y. wrote the manuscript and supervised the project. All authors read and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks Chung Hang Jonathan Choi, Anna Leopold and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Characterization of the mechanical fingerprint of the hairpin unfolding in the cRGD-S module using AFM.
a, Typical force-extension (FE) curves between integrin-functionalized tip with surface modified with no cRGD conjugated S (nRGD-S), cRGD-S, and cRGD-S with absent hairpin structure (cRGD-Sh-), respectively. For multiple pulling experiments, the hairpin was allowed to refold after one pulling cycle for 5 min, followed by the next pulling cycle. Red and blue peaks represent hairpin unfolding and cantilever unbinding, respectively. b, Left: Quantitative analysis for cantilever unbinding probabilities, calculated as the number of curves with unbinding peaks (the blue peak in the FE curve) divided by the total number of FE curves. Right: Quantitative analysis for hairpin unfolding probabilities relative to FE curves with the specific unbinding peak, calculated as the number of curves with hairpin unfolding peaks (the red peak in the FE curve) divided by the total number of curves with cantilever unbinding peaks (the blue peak in the FE curve). Data were measured from 126 curves from 3 independent experiments and shown as mean ± S.D. (n = 3). According to the probability kinetics model of 2D reactions, it is usually necessary to maintain a lower cantilever unbinding probability to obtain reliable single-molecule interaction data. Therefore, 20-25% unbinding probability in this study allows for a high probability of over 80% for single-molecule interactions, ensuring effective experimental condition control.
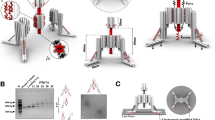
Extended Data Fig. 2 Worm-like chain (WLC) model analysis.
a, A typical FE curve (blue) and its theoretical fitting curve (red) based on the WLC model (The details please see Supplementary Note 3 in the supplementary information). b, Table of the values of the fitted persistence length Lp and calculated total free energy (ΔG). The persistence length Lp was fitted using WLC model from 60 curves from 3 independent experiments and presented as mean ± S.D. c, Schematic illustration of the approach-retraction process, delineating the reaction state at each stage. Within one approach and retraction cycle, the cantilever tip contacts the substrate-attached molecule cRGD-S, advancing into a repulsive contact regime until a compression force of 500 pN is reached. This phase is succeeded by the retraction of the tip. As retraction begins, the force initially reduces to a zero point, marking the start of the red hairpin unfolding region (Point A). As the distance between the substrate and cantilever increases, the S hairpin elongates, and the resulting decrease in its entropy generates a restoring force that bends the cantilever, depicted by the first small peak (Point B). Upon the unfolding of the hairpin domain, the DNA’s contour length extends, bringing the force on the cantilever back to a near zero point. This juncture is noted as both the end of the red hairpin unfolding region and the start of the blue cantilever unbinding region (Point C). The further extension applies force on the cantilever again until the detachment of the unfolded S molecule from the AFM tip takes place, indicated by the second peak (Point D). Eventually, the force on the cantilever returns to a near zero point, marking the end of the blue cantilever unbinding region (Point E).
Extended Data Fig. 3 Characterization of F-TSDR by AFM.
a, Typical FE curves between the integrin-functionalized tip and cRGD-S modified surface in the presence of excess soluble free T modules or the control T modules with Tme. The contact between the integrin-functionalized tip and Sf/Sh/Te triplex-modified surface, the expected product of F-TSDR, was set as a positive control event. b, Schematic illustration of the experimental testing, data collection and probability calculation processes. Quantitative analysis for hairpin unfolding probabilities relative to FE curves with the specific unbinding peak, calculated as the number of curves with hairpin unfolding peaks (the red peak in the FE curve) divided by the total number of curves with cantilever unbinding peaks (the blue peak in the FE curve). Blue bars represent the hairpin unfolding probabilities for the cRGD-S-modified surface. Fuchsia bars represent the hairpin unfolding probabilities of the cRGD-S-modified surface in the presence or absence of T and Tme modules. Data were measured from 30 curves from 3 independent experiments. Data represent mean ± S.D. (n = 3), and significance was determined by unpaired two-tailed Student’s t−test (NS, not significant). Before indicated treatments, the probability of hairpin unfolding among the three groups only varies within the range of 70% to 90%. This small variation in the hairpin unfolding probability can be attributed to the two factors, the inherently uncertainties associated with AFM as a single-molecule detection technique and potential system errors. The calculations through t-test show no statistically significant differences among the three groups as well as the samples before and after blank/Tme addition, whereas, after the addition of T, the probability of hairpin unfolding dramatically drops to ~20%. This significant difference sufficiently demonstrates the marked change in the probability of the hairpin unfolding after T addition, validating that the product of F-TSDR, Sf/Sh/Te triplex, prevents the unfolded hairpin from refolding.
Extended Data Fig. 4 Single colour dSTORM imaging of the cell surface distribution of c-Met, S, and T, respectively, and dual-colour dSTORM imaging for nanodomain analysis of S and T on a same cell.
a, A549 cells were fixed and stained with different conditions: (i) with primary c-Met antibody followed by Alexa Fluor 647-labelled secondary antibody, (ii) with Alexa Fluor 532-labelled S, and (iii) with Alexa Fluor 647-labelled T. Scale bars represent 5 μm in the primary images and 1 μm in the zoomed-in images. b, The histogram showcases the frequency distribution of the different nanodomain sizes. Data is derived from 130 random nanodomains across 3 cells in three independent experiments. c, Schematic illustration of the dual-colour dSTORM imaging to visualize the nanodomains in the same cell labelled by S and T, both having comparable dissociation constants (shown in Supplementary Fig. 13 and 14). d, A549 cells underwent staining using Alexa Fluor 532-labelled S and Alexa Fluor 647-labelled T and subsequent fixation. The reconstructed dSTORM images for both Alexa Fluor 532-labelled S and Alexa Fluor 647-labelled T in a representative single cell were displayed. Scale bars indicate 5 μm in the original images and 1 μm in the magnified images. e, The graph depicts the average number of nanodomains per µm². f, The graph depicts the typical nanodomain areas. The data in e (n = 3) and f (n = 130) derive from 18 randomly chosen regions of interest (ROIs) with an area of 2 × 2 µm² from three cells in three independent experiments (a total of 130 nanodomains) and are presented as the mean ± S.D. There was no significant difference in the nanodomains of S and T captured in both channels, as determined by an unpaired two-tailed Student’s t-test (NS, not significant).
Extended Data Fig. 5 Co-localization analysis of S and T using Dual-Colour dSTORM Imaging.
a, A representative merged dual-colour dSTORM image showing the distribution of Alexa Fluor 532-labelled S and Alexa Fluor 647-labelled T on a single A549 cell. Two specific ROIs measuring 4 × 4 µm², labelled as (i) and (ii), are enlarged to highlight co-localization details. Further magnification illustrates three types of co-localization: full overlap, partial overlap, and edge overlap within individual nanodomains. The scale bars represent 5 μm for the main image, 1 μm for the enlarged ROIs, and 200 nm for the individual nanodomain images. b, For every dual-colour dSTORM image, 18 stochastically chosen ROIs of 3 × 3 µm² were extracted from three cells across three independent experiments (a total of 230 nanodomains) to analyse the spatial association between S and T positive nanodomains. The graph displays the co-localization relationships, presenting the percentages of isolated versus the overlapped nanodomains, with the overlaps categorized as full overlap, partial overlap and edge overlap. Data are presented as mean ± S.D. (n = 18). c, Further proportionate analysis of the overlapped nanodomains reveals the distribution among the three aforementioned overlap scenarios: full, partial, and edge overlap. These proportions are presented as mean ± S.D. (n = 18). According to the dual-colour dSTORM analysis, around 84% of the nanodomains (with diameters ranging 50 nm to 200 nm) showed overlap. Within these overlapped nanodomains of S-positive and T-positive signals, about 33% showed full overlap, 24% had partial overlap, and 27% demonstrated edge overlap. This analysis implies that on the entire membrane of a cell, 33% of S and T are colocalized and 51% of S and T are adjacent within the nanodomains. The details please see Supplementary Note 2 in the supplementary information.
Extended Data Fig. 6 Visualization of cRGD/c-Met AMR system upon sensing and transmitting integrin-mediated intercellular force on Hela cells.
a, Representative images of brightfield, Cy5, FAM and TAMRA channels for the Hela cells treated with no cRGD conjugated AMR (nRGD/c-Met AMR) or cRGD/c-Met AMR (400 nM). For the actomyosin inhibition, Hela cells were pre-treated with Blebbistatin (10 µM) for 10 min before the addition of cRGD/c-Met AMR. Scale bar, 10 µm. b, Quantification of the relative FAM/Cy5 and TAMRA/Cy5 ratio to characterize the F-TSDR at cell-cell junctions. Data represent mean ± S.D. from independent experiments (n = 10). Significance was determined by unpaired two-tailed Student’s t-test.
Extended Data Fig. 7 The performance of AMR on isolated single-cell and multicell colony was examined using a high-density single-cell microarray.
a, Schematic illustration of the fabrication of the microarray for visualization of GMD performance on isolated single-cell or multicell aggregation. The microarray was fabricated by attaching a PDMS stencil layer onto a flat glass surface of confocal dishes. b, Representative images of randomly chosen microwells with single-cell occupancy and multicell colony were presented. Successful AMR functionalization was visualized using Cy5 fluorescence as a reference signal. The force-sensing signal and force-transmitting signal are shown in FAM and TAMRA channels. Scale bars, 10 µm. c, To characterize the performance of the AMR, the force-sensing FAM/Cy5 ratios and force-transmitting TAMRA/Cy5 ratios were quantified from 150 regions of interest (ROIs) of 30 randomly chosen wells from three independent experiments. The graphs presented the frequency of occurrence of these ratios for isolated single cells and the cell-cell junctions in multi-colonies. A number of examples of the representative images of randomly chosen microwells with single-cell occupancy and multi-cell colony were presented in Supplementary Fig. 16. All of the isolated single cells exhibited obviously the reference Cy5 signals of AMR, indicating the successful functionalization of AMR on cell membrane, but neither force-sensing (FAM) nor transmitting (TAMRA) signals. In contrast, multi-cellular colonies manifested distinct FAM and TAMRA signals of AMR at cell-cell contacts. This data indicates that the operation of AMRs is fundamentally dependent on the forces exerted by the integrin receptors of adjacent cells, thereby highlighting the functionality of AMR in trans intercellular integrin-mediated mechanic interactions over cis integrin engagements within individual cells.
Extended Data Fig. 8 Visualization of F-TSDR and ERK response activity of living cells.
a, Schematic illustration of the ERK-KTR fused with the Clover protein. The ERK-KTRs are composed of an engineered construct in which a kinase substrate is fused to a bipartite nuclear localization signal (bNLS) and nuclear export signal (NES). The phosphorylation of the substrate suppresses bNLS activity and enhances NES activity, leading to cytoplasmic translocation. The shutdown of cell signalling pathways leads to the dephosphorylation of the substrate, which deactivates NES activity and recovers bNLS activity, leading to nuclear translocation. b, Hela cells were transfected with ERK-KTR for 24 h, followed by treatment of cRGD/c-Met AMR (400 nM) or HGF (2 nM). Cells were pre-treated with 100 nM Foretinib for 2 h to inhibit the kinase activity of c-Met. Time-lapsed images were collected to track the ERK-KTR translocation and TAMRA fluorescence change. The arrows indicate the cells experiencing nucleus-to-cytosol translocation of ERK-KTR. Scale bar, 10 µm. c, Sequential imaging captured from cRGD/c-Met AMR-modified HeLa cells post-transfection with ERK-KTR plasmids. Displayed images from the brightfield, Clover, and TAMRA fluorescence channels are colour-coded in gray, green, and red, respectively. Scale bars, 10 µm. d, Normalized ratio of the fluorescence intensities of ERK-KTR-Clover in the cytosol and nucleus are shown. Data represent mean ± S.D. (n = 3 from three independent experiments). The calculation of the ERK response ratio is calculated by dividing the number of cells with positive ERK translocation by the number of cells with Clover-positive. Noteworthy, nearly all of the cell with Clover-positive are accompanied by AMR activation signal, which illustrated the correlation between force-induced AMR activation at cell-cell junctions and the ERK activation. The translocation of the ERK reporter to the cytosol commenced and peaked at approximately 30 minutes. Following this peak, a gradual decline in cytosolic translocation was observed, which returned to baseline levels at around 60 minutes.
Extended Data Fig. 9 Customized AMR with responsiveness to the E-cadherin-mediated force input and CI-M6PR endocytosis force input, respectively.
a, Representative images for the nE-cad/c-Met AMR or E-cad/c-Met AMR-grafted A549 cells treated with or without Blebbistatin (10 μM) or EGTA (5 mM) are shown in the brightfield, Cy5, FAM, and TAMRA channels. Scale bars, 10 µm. b, Western blotting showed the c-Met phosphorylation (Tyr1234/Tyr1235) and ERK phosphorylation (Thr202/Tyr204) in the lysates of A549 cells with indicated treatments. The α-tubulin was used as an internal reference. E-cad/c-Met AMRS represent the control in the absence of AMR-T. c, Representative images for the Hela cells engineered with 400 nM nCI-M6PR/c-Met AMR, CI-M6PR/c-Met AMR with random ligand sequence, and CI-M6PR/c-Met AMR, respectively, are shown in brightfield, Cy5, FAM and TAMRA channels. For the endocytosis inhibition, cells were pre-treated with Pitstop (30 µM) for 30 min before CI-M6PR/c-Met AMR functionalization. For the ligand competition control, cells were pre-treated with CI-M6PR aptamers (1 µM) for 30 min before CI-M6PR/c-Met AMR functionalization. Scale bar, 10 µm. d, Western blotting showed the c-Met phosphorylation (Tyr1234/Tyr1235) and ERK phosphorylation (Thr202/Tyr204) in the lysates of Hela cells with indicated treatments. The α-tubulin was used as an internal reference. CI-M6PR/c-Met AMRS represent the control in the absence of AMR-T.
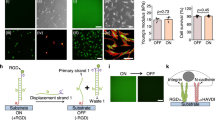
Extended Data Fig. 10 The versatility of AMR to rewire integrin-mediated force input to promote FGFR1 signalling, and E-cadherin-mediated force input to promote FGFR1 signalling in neural stem cell maintenance, respectively.
a, Representative images for the NIH3T3 cells engineered with 400 nM nRGD/FGFR1 AMR or cRGD/FGFR1 AMR are shown in brightfield, Cy5, FAM and TAMRA channels. For actomyosin inhibition, cells were pre-treated with Blebbistatin (10 µM) for 10 min before cRGD/FGFR1 AMR functionalization. Scale bar, 10 µm. b, Western blotting of FGFR1 phosphorylation (Tyr653/Tyr654) and ERK phosphorylation (Thr202/Tyr204) in the cell lysates of NIH3T3 cells with indicated treatments. The α-tubulin was used as an internal reference. cRGD/FGFR1 AMRS represent the control in the absence of AMR-T. c, Neural stem cells (NSCs) were functionalized with nE-cad/FGFR1 AMR and E-cad/FGFR1 AMR at a concentration of 400 nM for 30 min. For the E-cadherin inhibition assay, cells were pre-treated with 5 mM EGTA for 30 min before E-cad/FGFR1 AMR functionalization. Following the incubation, cells were visualized using CLSM and representative images captured from brightfield, Cy5, FAM, and TAMRA channels are presented. Scale bar, 10 µm. d, Western blotting of FGFR1 phosphorylation (Tyr653/Tyr654) and ERK phosphorylation (Thr202/Tyr204) in the cell lysates of NSCs with indicated treatments. The α-tubulin was used as an internal reference. E-cad/FGFR1 AMRS represent the control in the absence of AMR-T.
Supplementary information
Supplementary Information
Supplementary Figs. 1–27, Tables 1–8, Notes 1–6, References and source data for unprocessed blots and gels.
Supplementary Data 1
Statistical source data for Supplementary figures.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 4
Unprocessed western blots.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Unprocessed western blots.
Source Data Extended Data Fig. 10
Unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, S., Wang, M., Tian, D. et al. DNA-functionalized artificial mechanoreceptor for de novo force-responsive signaling. Nat Chem Biol (2024). https://doi.org/10.1038/s41589-024-01572-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41589-024-01572-x
This article is cited by
-
Forced rewiring of RTK signaling
Nature Chemical Biology (2024)