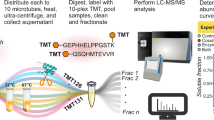
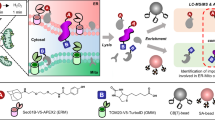
Abstract
Metal-binding proteins (MBPs) have various and important biological roles in all living species and many human diseases are intricately linked to dysfunctional MBPs. Here, we report a chemoproteomic method named ‘metal extraction-triggered agitation logged by thermal proteome profiling’ (METAL-TPP) to globally profile MBPs in proteomes. The method involves the extraction of metals from MBPs using chelators and monitoring the resulting protein stability changes through thermal proteome profiling. Applying METAL-TPP to the human proteome with a broad-spectrum chelator, EDTA, revealed a group of proteins with reduced thermal stability that contained both previously known MBPs and currently unannotated MBP candidates. Biochemical characterization of one potential target, glutamine-fructose-6-phosphate transaminase 2 (GFPT2), showed that zinc bound the protein, inhibited its enzymatic activity and modulated the hexosamine biosynthesis pathway. METAL-TPP profiling with another chelator, TPEN, uncovered additional MBPs in proteomes. Collectively, this study developed a robust tool for proteomic discovery of MBPs and provides a rich resource for functional studies of metals in cell biology.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The MS proteomics data were deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the iProX partner repository with the dataset identifier PXD024925 for EDTA and PXD029856 for TPEN. Source data are provided with this paper.
Code availability
TPP data preprocessing and the fitting of melting curves were performed using custom algorithms (https://doi.org/10.5281/zenodo.10278871).
References
Hagedoorn, P. L. Microbial metalloproteomics. Proteomes 3, 424–439 (2015).
Telpoukhovskaia, M. A. & Orvig, C. Werner coordination chemistry and neurodegeneration. Chem. Soc. Rev. 42, 1836–1846 (2013).
Roberts, E. A. & Sarkar, B. Metalloproteomics: focus on metabolic issues relating to metals. Curr. Opin. Clin. Nutr. Metab. Care 17, 425–430 (2014).
Lothian, A. et al. Metalloproteomics: principles, challenges and applications to neurodegeneration. Front. Aging Neurosci. 5, 35 (2013).
Zeng, X., Cheng, Y. & Wang, C. Global mapping of metalloproteomes. Biochemistry 60, 3507–3514 (2021).
Cvetkovic, A. et al. Microbial metalloproteomes are largely uncharacterized. Nature 466, 779–782 (2010).
Smith, S. D., She, Y. M., Roberts, E. A. & Sarkar, B. Using immobilized metal affinity chromatography, two-dimensional electrophoresis and mass spectrometry to identify hepatocellular proteins with copper-binding ability. J. Proteome Res. 3, 834–840 (2004).
Sevcenco, A. M. et al. Exploring the microbial metalloproteome using MIRAGE. Metallomics 3, 1324–1330 (2011).
Pace, N. J. & Weerapana, E. A competitive chemical–proteomic platform to identify zinc-binding cysteines. ACS Chem. Biol. 9, 258–265 (2014).
Bak, D. W. & Weerapana, E. Monitoring Fe–S cluster occupancy across the E. coli proteome using chemoproteomics. Nat. Chem. Biol. 19, 356–366 (2023).
Miki, T. et al. A conditional proteomics approach to identify proteins involved in zinc homeostasis. Nat. Methods 13, 931–937 (2016).
Andreini, C., Bertini, I. & Rosato, A. Metalloproteomes: a bioinformatic approach. Acc. Chem. Res. 42, 1471–1479 (2009).
Molina, D. M. & Nordlund, P. The cellular thermal shift assay: a novel biophysical assay for in situ drug target engagement and mechanistic biomarker studies. Annu. Rev. Pharmacol. Toxicol. 56, 141–161 (2016).
Martinez Molina, D. et al. Monitoring drug target engagement in cells and tissues using the cellular thermal shift assay. Science 341, 84–87 (2013).
Savitski, M. M. et al. Tracking cancer drugs in living cells by thermal profiling of the proteome. Science 346, 1255784 (2014).
Tan, C. S. H. et al. Thermal proximity coaggregation for system-wide profiling of protein complex dynamics in cells. Science 359, 1170–1176 (2018).
Huber, K. V. M. et al. Proteome-wide drug and metabolite interaction mapping by thermal-stability profiling. Nat. Methods 12, 1055–1057 (2015).
Huang, J. X. et al. High throughput discovery of functional protein modifications by hotspot thermal profiling. Nat. Methods 16, 894–901 (2019).
Potel, C. M. et al. Impact of phosphorylation on thermal stability of proteins. Nat. Methods 18, 757–759 (2021).
Smith, I. R. et al. Identification of phosphosites that alter protein thermal stability. Nat. Methods 18, 760–762 (2021).
Becher, I. et al. Pervasive protein thermal stability variation during the cell cycle. Cell 173, 1495–1507 (2018).
Mateus, A. et al. The functional proteome landscape of Escherichia coli. Nature 588, 473–478 (2020).
Sedlak, E., Zoldak, G. & Wittung-Stafshede, P. Role of copper in thermal stability of human ceruloplasmin. Biophys. J. 94, 1384–1391 (2008).
Bonomi, F., Fessas, D., Iametti, S., Kurtz, D. M. Jr. & Mazzini, S. Thermal stability of Clostridium pasteurianum rubredoxin: deconvoluting the contributions of the metal site and the protein. Protein Sci. 9, 2413–2426 (2000).
Fish, A., Danieli, T., Ohad, I., Nechushtai, R. & Livnah, O. Structural basis for the thermostability of ferredoxin from the cyanobacterium Mastigocladus laminosus. J. Mol. Biol. 350, 599–608 (2005).
Crepin, T. et al. Mutational and metal binding analysis of the endonuclease domain of the influenza virus polymerase PA subunit. J. Virol. 84, 9096–9104 (2010).
Botelho, H. M., Koch, M., Fritz, G. & Gomes, C. M. Metal ions modulate the folding and stability of the tumor suppressor protein S100A2. FEBS J. 276, 1776–1786 (2009).
Scolnick, L. R., Kanyo, Z. F., Cavalli, R. C., Ash, D. E. & Christianson, D. W. Altering the binuclear manganese cluster of arginase diminishes thermostability and catalytic function. Biochemistry 36, 10558–10565 (1997).
Kanyo, Z. F., Scolnick, L. R., Ash, D. E. & Christianson, D. W. Structure of a unique binuclear manganese cluster in arginase. Nature 383, 554–557 (1996).
Hebert, L. F. et al. Overexpression of glutamine:fructose-6-phosphate amidotransferase in transgenic mice leads to insulin resistance. J. Clin. Invest. 98, 930–936 (1996).
Srinivasan, V. et al. Glutamine fructose-6-phosphate amidotransferase (GFAT) gene expression and activity in patients with type 2 diabetes: inter-relationships with hyperglycaemia and oxidative stress. Clin. Biochem. 40, 952–957 (2007).
Kim, J. et al. The hexosamine biosynthesis pathway is a targetable liability in KRAS/LKB1 mutant lung cancer. Nat. Metab. 2, 1401–1412 (2020).
Nakata, M., O’Rourke, R., Wilson, S., Chilson, K. & Selitrennikoff, C. P. A novel assay for fungal ketol-isomerase activity. J. Antibiot. 54, 737–743 (2001).
Oliveira, I. A. et al. Enzymatic and structural properties of human glutamine:fructose-6-phosphate amidotransferase 2 (hGFAT2). J. Biol. Chem. 296, 100180 (2021).
Kroef, V. et al. GFPT2/GFAT2 and AMDHD2 act in tandem to control the hexosamine pathway. eLife 11, e69223 (2022).
Ruegenberg, S. et al. Loss of GFAT-1 feedback regulation activates the hexosamine pathway that modulates protein homeostasis. Nat. Commun. 11, 687 (2020).
Milewski, S. Glucosamine-6-phosphate synthase—the multi-facets enzyme. Biochim. Biophys. Acta 1597, 173–192 (2002).
Mouilleron, S., Badet-Denisot, M. A. & Golinelli-Pimpaneau, B. Glutamine binding opens the ammonia channel and activates glucosamine-6P synthase. J. Biol. Chem. 281, 4404–4412 (2006).
Mouilleron, S., Badet-Denisot, M. A. & Golinelli-Pimpaneau, B. Ordering of C-terminal loop and glutaminase domains of glucosamine-6-phosphate synthase promotes sugar ring opening and formation of the ammonia channel. J. Mol. Biol. 377, 1174–1185 (2008).
Radford, R. J. & Lippard, S. J. Chelators for investigating zinc metalloneurochemistry. Curr. Opin. Chem. Biol. 17, 129–136 (2013).
Shumaker, D. K., Vann, L. R., Goldberg, M. W., Allen, T. D. & Wilson, K. L. TPEN, a Zn2+/Fe2+ chelator with low affinity for Ca2+, inhibits lamin assembly, destabilizes nuclear architecture and may independently protect nuclei from apoptosis in vitro. Cell Calcium 23, 151–164 (1998).
Aron, A. T., Ramos-Torres, K. M., Cotruvo, J. A. & Chang, C. J. Recognition- and reactivity-based fluorescent probes for studying transition metal signaling in living systems. Acc. Chem. Res. 48, 2434–2442 (2015).
Perrin, J. et al. Identifying drug targets in tissues and whole blood with thermal-shift profiling. Nat. Biotechnol. 38, 303–308 (2020).
Frey, A. G. & Eide, D. J. Roles of two activation domains in Zap1 in the response to zinc deficiency in Saccharomyces cerevisiae. J. Biol. Chem. 286, 6844–6854 (2011).
Shirley, M. & Plosker, G. L. Deferasirox: a review of its use for chronic iron overload in patients with non-transfusion-dependent thalassaemia. Drugs 74, 1017–1027 (2014).
Szentmihalyi, K. Metal element homeostasis and oxidative stress in pathological processes. Orv. Hetil. 160, 1407–1416 (2019).
Dayani, P. N., Bishop, M. C., Black, K. & Zeltzer, P. M. Desferoxamine (DFO)-mediated iron chelation: rationale for a novel approach to therapy for brain cancer. J. Neurooncol. 67, 367–377 (2004).
Brazier, M. W. et al. Manganese chelation therapy extends survival in a mouse model of M1000 prion disease. J. Neurochem. 114, 440–451 (2010).
Ding, X., Xie, H. & Kang, Y. J. The significance of copper chelators in clinical and experimental application. J. Nutr. Biochem. 22, 301–310 (2011).
Becher, I. et al. Thermal profiling reveals phenylalanine hydroxylase as an off-target of panobinostat. Nat. Chem. Biol. 12, 908–910 (2016).
Schnaars, C. et al. Synthesis and preclinical evaluation of TPA-based zinc chelators as metallo-β-lactamase inhibitors. ACS Infect. Dis. 4, 1407–1422 (2018).
Piazza, I. et al. A map of protein-metabolite interactions reveals principles of chemical communication. Cell 172, 358–372 (2018).
Broschat, K. O. et al. Kinetic characterization of human glutamine-fructose-6-phosphate amidotransferase I: potent feedback inhibition by glucosamine 6-phosphate. J. Biol. Chem. 277, 14764–14770 (2002).
Reinhard, F. B. et al. Thermal proteome profiling monitors ligand interactions with cellular membrane proteins. Nat. Methods 12, 1129–1131 (2015).
Virtanen, P. et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat. Methods 17, 261–272 (2020).
Waldron, K. J. & Robinson, N. J. How do bacterial cells ensure that metalloproteins get the correct metal? Nat. Rev. Microbiol. 7, 25–35 (2009).
Mccoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Teplyakov, A., Obmolova, G., Badet-Denisot, M. A., Badet, B. & Polikarpov, I. Involvement of the C terminus in intramolecular nitrogen channeling in glucosamine 6-phosphate synthase: evidence from a 1.6 angstrom crystal structure of the isomerase domain. Structure 6, 1047–1055 (1998).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Acknowledgements
We thank the Computing Platform of the Center for Life Science for supporting the proteomics data analysis and Analytical Instrumentation Center of College of Chemistry and Molecular Engineering, Peking University, for ICP-MS analysis. We thank the National Center for Protein Sciences at Peking University for assistance with protein crystallization and the staff of the Shanghai Synchrotron Radiation Facility and KEK Photon Factory for assistance with X-ray data collection. We thank the National Center for Protein Sciences at Peking University for assistance with the ITC experiment. We thank the Metabolomics Facility Center of Metabolomics and Lipidomics in the National Protein Science Technology Center of Tsinghua University for LC–MS/MS experiments of UDP-GlcNAc quantification. This work was supported by the National Natural Science Foundation of China (no. 21925701, no. 92153301 and no. 91953109) to C.W.
Author information
Authors and Affiliations
Contributions
X.Z., W.Q. and C.W. conceptualized the project. X.Z. and X.W. conducted most of the experiments unless otherwise specified. T.W. solved the structure under the guidance of J.X. and purified GFPT1, GFPT2 and GPATCH11. Y.L. developed computational algorithms to analyze the TMT proteomic data. Z.T., Y.Z. and T.F. helped to carry out the thermal stability assay of GlmS-C, performed the activity assay of GlmS and constructed certain mutants. Y.C. and F.W. helped with data extraction and computational analysis. B.M. helped with the ICP-MS measurement. C.G. helped to refine the structure. X.Z., X.W. and C.W. analyzed the data and wrote the paper with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks Mikhail Savitski and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Gel-based validation of METALL-TPP with EDTA on purified proteins and in HeLa cell lysates.
a, Four human proteins were recombinantly expressed and purified from E. coli. The experiments were repeated two times with similar results. b, The extraction of zinc from SORD by EDTA as measured by ICP-MS. c, Optimization of the EDTA concentrations to perturb SORD’s thermal stability in 2 mg/mL HeLa cell lysates by immunoblotting. GAPDH was used as a negative control. d, 4 mM EDTA treatment of HeLa cell lysates (2 mg/mL) does not affect the global proteome stability as demonstrated by SDS-PAGE stained with Coomassie Brilliant Blue (CBB). In b, error bars represent mean ± s.d. Results are from three independent experiments. Statistical differences were determined by a two-sided Student’s t-test. In c and d, the experiments were repeated two and three times with similar results, respectively.
Extended Data Fig. 2 Thermal shift curves of representative proteins quantified from the MS-based METAL-TPP profiling experiment with EDTA.
Annotated MBPs include SORD, ENO1, GSN, TRAF2 and SEPTIN2. Two novel MBP candidate proteins are RSU1 and GFPT2. GAPDH is the non-MBPs control. For each protein, the thermal shift curves were quantified from the TMT-based quantification and the Tm values were fitted by GraphPad Prism 7.0. Error bars represent mean ± s.d. Results are from three independent experiments. Statistical differences were determined by a two-sided Student’s t-test.
Extended Data Fig. 3 Analysis of proteins quantified from the METAL-TPP profiling with EDTA.
a, Analysis of the subcellular locations of those annotated MBPs found in different thermal stability groups. b, Analysis of the secondary structure content of those annotated MBPs found in different thermal stability groups. For each group, the percentage of helix, sheet and loop contents are shown. The values of each box and whisker are maximum value, upper quartile, median, lower quartile and minimum value. The cellular compartment (c) and molecular function (d) analysis of the 165 thermally stabilized proteins regardless they are annotated MBPs or not. e, Distribution of ΔTm values for annotated zinc-binding and magnesium-binding MBPs (‘Zn-MBPs’ and ‘Mg-MBPs’) quantified from the METAL-TPP profiling experiments. ΔTm values for Zn-MBPs, Mg-MBPs and all other MBPs are shown in cyan, orange and grey dots, respectively. Moving averages (window of 20) of Zn-and Mg-MBPs are shown as cyan and orange lines. All data were generated from three (n = 3) independent biological replicates. Statistical differences were determined by a two-sided Student’s t-test.
Extended Data Fig. 4 Biochemical characterization of purified GFPT1 and GFPT2.
a, SDS-PAGE of GFPT1 and GFPT2 with internal 6xHis tags that were purified from insect cells after nickel chromatography. The TEV protease (‘TEV’) was purified in parallel as a negative control. The purified proteins were further filtered to remove excessive imidazole in the buffer. b, Size exclusion chromatography (SEC) of the purified GFPT2. The protein came out as one single aggregation peak. c, Size exclusion chromatography (SEC) of the purified GFPT1. The protein came out as one aggregation peak and one active-form peak, the latter of which was resolved by SDS-PAGE. d, Measurement of metal content of the purified GFPT1 after SEC by ICP-MS. Significant zinc-binding activity was detected. In a and c, the experiment was repeated two times with similar results. In d, error bars represent mean ± s.d. Results are from three independent experiments. Statistical differences were determined by a two-sided Student’s t-test.
Extended Data Fig. 5 Biochemical characterization of recombinant GLMS as a zinc-binding protein.
a, Structures of GLMS with the two substrates bound (PDB:4AMV & PDB:1XFF). Based on these structures, glutamine (green sphere) is bound at the N-terminal domain (red ribbon) and fructose-6-P (pink sphere) was bound at the C-terminal domain (blue ribbon). b, Recombinant expression and purification of the full-length GLMS from E. coli with a GST tag. Samples were collected along the purification process and analyzed by SDS-PAGE including supernatant after cell lysis (S), flow through after GST columns (FT), three wash steps (W1, W2 and W3), elution from GST columns by 20 mM glutathione (E) and GST tag was cleaved by TEV protease (GLMS). Asterisk marks the position of purified GLMS. c, The binding affinity of zinc with GLMS was measured as 18 ± 5 μM by ITC. During measurement, 1 mM ZnSO4 (fresh) was titrated to 25 μM GLMS in 25 mM Tris buffer. d, Recombinant expression and purification of GLMS-C from E. coli with the 6xHis tag. The His tag was cleaved by TEV protease. In a and d, the experiment was repeated two times with similar results.
Extended Data Fig. 6 Testing of potential zinc-binding site on GFPT1/2 that is equivalent to C301 in GLMS.
a, GFPT2-C375A is more sensitive to zinc inhibition as compared to the wild-type (WT) GFPT2. Hela cells were transiently overexpressed with WT or C375A mutant of GFPT2 and then treated with 100 μM zinc for 30 min. After lysis, the enzymatic activity of GFPT2 were measured (n = 3). b, Zinc treatment similarly reduces the HBP flux of in both WT- and C375A-overexpressing cells. Quantitative LC-MS/MS analysis showed decreased UDP-GlcNAc levels after HeLa cells overexpressing GFPT2 WT and C375A mutant were treated with 100 μM zinc for 30 min (n = 3). c, Purified WT-GFPT1 and the C374A mutant were equally sensitive toward zinc inhibition. Recombinant WT-GFPT1 and the C374A mutant were treated with different concentration of zinc, followed by the measurement of their activity (n = 3). In a-c, error bars represent mean ± s.d. Results are from three independent experiments. Statistical differences were determined by a two-sided Student’s t-test.
Extended Data Fig. 7 Comparison of the METAL-TPP profiling results with TEPN and EDTA.
a, Overlap of MBPs with reduced thermal stability from the TPEN and EDTA datasets. b, Analysis of 37 MBPs identified both by the TPEN and EDTA profiling. c, Analysis of MBPs identified only in the TPEN dataset. d, Analysis of MBPs identified only in the EDTA dataset. All data were generated from three (n = 3) independent biological replicates.
Extended Data Fig. 8 Characterization of GPATCH11 as a zinc-binding protein.
a, EDTA treatment decreases the thermal stability of GPATCH11 in HeLa cell lysates. Thermal shift curves of GPATCH11 quantified from the MS-based METAL-TPP profiling experiment with EDTA treatment were shown. The Tm values were fitted by GraphPad Prism 7.0. Error bars represent mean ± s.d. Results are from three independent experiments. Statistical differences were determined by a two-sided Student’s t-test. b, SDS-PAGE of GPATCH11 purified from insect cells with a GST tag. Asterisk marks the position of GPATCH11-GST. The experiment was repeated two with similar results.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2.
Supplementary Data 1
List of Tm and ΔTm values for each protein quantified from three biological replicates of METAL-TPP experiments with EDTA.
Supplementary Data 2
List of 5,833 annotated MBPs extracted from UniProt.
Supplementary Data 3
List of proteins identified with significantly reduced thermal stability by METAL-TPP with EDTA.
Supplementary Data 4
List of Tm and ΔTm values for each protein quantified from three biological replicates of METAL-TPP experiments.
Source data
Source Data Fig. 1
Unprocessed gels.
Source Data Fig. 2
Unprocessed gels.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Unprocessed gels and statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 1
Unprocessed gels and statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
ITC data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zeng, X., Wei, T., Wang, X. et al. Discovery of metal-binding proteins by thermal proteome profiling. Nat Chem Biol (2024). https://doi.org/10.1038/s41589-024-01563-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41589-024-01563-y