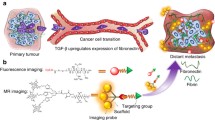
Abstract
Purpose
Pancreatic ductal adenocarcinoma (PDAC) is a lethal hypovascular tumor surrounded by dense fibrosis. Albumin-bound paclitaxel and gemcitabine (AG) chemotherapy is the mainstay of PDAC treatment through depleting peritumoral fibrosis and killing tumor cells; however, it remains challenging due to the lack of a noninvasive imaging method evaluating fibrotic changes during AG chemotherapy. In this study, we developed a dual-modality imaging platform that enables noninvasive, dynamic, and quantitative assessment of chemotherapy-induced fibrotic changes through near-infrared fluorescence molecular imaging (FMI) and magnetic resonance imaging (MRI) using an extradomain B fibronectin (EDB-FN)-targeted imaging probe (ZD2-Gd-DOTA-Cy7).
Methods
The ZD2-Gd-DOTA-Cy7 probe was constructed by conjugating a peptide (Cys-TVRTSAD) to Gd-DOTA and the near-infrared dye Cy7. PDAC murine xenograft models were intravenously injected with ZD2-Gd-DOTA-Cy7 at a Gd concentration of 0.05 mmol/kg or free Cy7 and Gd-DOTA as control. The normalized tumor background ratio (TBR) on FMI and the T1 reduction ratio on MRI were quantitatively analyzed. For models receiving AG chemotherapy or saline, MRI/FMI was performed before and after treatment. Histological analyses were performed for validation.
Results
The ZD2-Gd-DOTA-Cy7 concentration showed a linear correlation with the fluorescence intensity and T1 relaxation time in vitro. The optimal imaging time was 30 min after injection of the ZD2-Gd-DOTA-Cy7 (0.05 mmol/kg), only half of the clinic dosage of gadolinium. Additionally, ZD2-Gd-DOTA-Cy7 generated a 1.44-fold and 1.90-fold robust contrast enhancement compared with Cy7 (P < 0.05) and Gd-DOTA (P < 0.05), respectively. For AG chemotherapy monitoring, the T1 reduction ratio and normalized TBR in the fibrotic tumor areas were significantly increased by 1.99-fold (P < 0.05) and 1.78-fold (P < 0.05), respectively, in the control group compared with those in the AG group.
Conclusion
MRI/FMI with a low dose of ZD2-Gd-DOTA-Cy7 enables sensitive imaging of PDAC and the quantitative assessment of fibrotic changes during AG chemotherapy, which shows potential clinical applications for precise diagnosis, post-treatment monitoring, and disease management.







Similar content being viewed by others
Data availability
Data are available on request from the authors.
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. https://doi.org/10.3322/caac.21708
Willett CG, Czito BG, Bendell JC, Ryan DP. Locally advanced pancreatic cancer. J Clin Oncol. 2005;23:4538–44. https://doi.org/10.1200/JCO.2005.23.911
Thomas D, Radhakrishnan P. Tumor-stromal crosstalk in pancreatic cancer and tissue fibrosis. Mol Cancer. 2019;18:14. https://doi.org/10.1186/s12943-018-0927-5
Haqq J, Howells LM, Garcea G, Metcalfe MS, Steward WP, Dennison AR. Pancreatic stellate cells and pancreas cancer: current perspectives and future strategies. Eur J Cancer. 2014;50:2570–82. https://doi.org/10.1016/j.ejca.2014.06.021
Xu Z, Vonlaufen A, Phillips PA, Fiala-Beer E, Zhang X, Yang L, et al. Role of pancreatic stellate cells in pancreatic cancer metastasis. Am J Pathol. 2010;177:2585–96. https://doi.org/10.2353/ajpath.2010.090899
Erkan M, Michalski CW, Rieder S, Reiser-Erkan C, Abiatari I, Kolb A, et al. The activated stroma index is a novel and independent prognostic marker in pancreatic ductal adenocarcinoma. Clin Gastroenterol Hepatol. 2008;6:1155–61. https://doi.org/10.1016/j.cgh.2008.05.006
Amrutkar M, Aasrum M, Verbeke CS, Gladhaug IP. Secretion of fibronectin by human pancreatic stellate cells promotes chemoresistance to gemcitabine in pancreatic cancer cells. BMC Cancer. 2019;19:596. https://doi.org/10.1186/s12885-019-5803-1
Von Hoff DD, Ramanathan RK, Borad MJ, Laheru DA, Smith LS, Wood TE, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. 2011;29:4548–54. https://doi.org/10.1200/JCO.2011.36.5742
Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–703. https://doi.org/10.1056/NEJMoa1304369
Pancreatic Adenocarcinoma. Version 2.2023. NCCN Clinical Practice Guidelines in Oncology. https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf
Alvarez R, Musteanu M, Garcia-Garcia E, Lopez-Casas PP, Megias D, Guerra C, et al. Stromal disrupting effects of nab-paclitaxel in pancreatic cancer. Br J Cancer. 2013;109:926–33. https://doi.org/10.1038/bjc.2013.415
Erstad DJ, Sojoodi M, Taylor MS, Jordan VC, Farrar CT, Axtell AL, et al. Fibrotic response to neoadjuvant therapy predicts survival in pancreatic cancer and is measurable with collagen-targeted molecular MRI. Clin Cancer Res. 2020;26:5007–18. https://doi.org/10.1158/1078-0432.CCR-18-1359
Yamaguchi J, Yokoyama Y, Fujii T, Yamada S, Takami H, Kawashima H, et al. Results of a phase II study on the use of neoadjuvant chemotherapy (FOLFIRINOX or GEM/nab-PTX) for borderline-resectable pancreatic cancer (NUPAT-01). Ann Surg. 2022;275:1043–9. https://doi.org/10.1097/SLA.0000000000005430
Hu Y, Chi C, Wang S, Wang L, Liang P, Liu F, et al. A comparative study of clinical intervention and interventional photothermal therapy for pancreatic cancer. Adv Mater. 2017;29. https://doi.org/10.1002/adma.201700448
Watabe T, Liu Y, Kaneda-Nakashima K, Shirakami Y, Lindner T, Ooe K, et al. Theranostics targeting fibroblast activation protein in the tumor stroma: (64)Cu- and (225)Ac-labeled FAPI-04 in pancreatic cancer xenograft mouse models. J Nucl Med. 2020;61:563–9. https://doi.org/10.2967/jnumed.119.233122
Crosby D, Bhatia S, Brindle KM, Coussens LM, Dive C, Emberton M, et al. Early detection of cancer. Science. 2022;375:eaay9040. https://doi.org/10.1126/science.aay9040
Peng D, Du Y, Shi Y, Mao D, Jia X, Li H, et al. Precise diagnosis in different scenarios using photoacoustic and fluorescence imaging with dual-modality nanoparticles. Nanoscale. 2016;8:14480–8. https://doi.org/10.1039/c6nr03809c
Xiong K, Zhang H, Du Y, Tian J, Ding S. Identification of HDAC9 as a viable therapeutic target for the treatment of gastric cancer. Exp Mol Med. 2019;51:1–15. https://doi.org/10.1038/s12276-019-0301-8
Song G, Zheng X, Wang Y, Xia X, Chu S, Rao J. A magneto-optical nanoplatform for multimodality imaging of tumors in mice. ACS Nano. 2019;13:7750–8. https://doi.org/10.1021/acsnano.9b01436
Zhang W, Liang X, Zhu L, Zhang X, Jin Z, Du Y, et al. Optical magnetic multimodality imaging of plectin-1-targeted imaging agent for the precise detection of orthotopic pancreatic ductal adenocarcinoma in mice. EBioMedicine. 2022;80:104040. https://doi.org/10.1016/j.ebiom.2022.104040
Tirkes T, Yadav D, Conwell DL, Territo PR, Zhao X, Venkatesh SK, et al. Magnetic resonance imaging as a non-invasive method for the assessment of pancreatic fibrosis (MINIMAP): a comprehensive study design from the consortium for the study of chronic pancreatitis, diabetes, and pancreatic cancer. Abdom Radiol (NY). 2019;44:2809–21. https://doi.org/10.1007/s00261-019-02049-5
Higashi M, Tanabe M, Okada M, Furukawa M, Iida E, Ito K. Influence of fat deposition on T1 mapping of the pancreas: evaluation by dual-flip-angle MR imaging with and without fat suppression. Radiol Med. 2020;125:1–6. https://doi.org/10.1007/s11547-019-01087-9
Ding Y, Rao SX, Meng T, Chen C, Li R, Zeng MS. Usefulness of T1 mapping on Gd-EOB-DTPA-enhanced MR imaging in assessment of non-alcoholic fatty liver disease. Eur Radiol. 2014;24:959–66. https://doi.org/10.1007/s00330-014-3096-y
Chen W, Schilperoort M, Cao Y, Shi J, Tabas I, Tao W. Macrophage-targeted nanomedicine for the diagnosis and treatment of atherosclerosis. Nat Rev Cardiol. 2022;19:228–49. https://doi.org/10.1038/s41569-021-00629-x
Qiao P, Ayat NR, Vaidya A, Gao S, Sun W, Chou S, et al. Magnetic resonance molecular imaging of extradomain B fibronectin improves imaging of pancreatic cancer tumor xenografts. Front Oncol. 2020;10:586727. https://doi.org/10.3389/fonc.2020.586727
Han Z, Zhang S, Fujiwara K, Zhang J, Li Y, Liu J, et al. Extradomain-B fibronectin-targeted dextran-based chemical exchange saturation transfer magnetic resonance imaging probe for detecting pancreatic cancer. Bioconjug Chem. 2019;30:1425–33. https://doi.org/10.1021/acs.bioconjchem.9b00161
Qiao PL, Gargesha M, Liu Y, Laney VEA, Hall RC, Vaidya AM, et al. Magnetic resonance molecular imaging of extradomain B fibronectin enables detection of pancreatic ductal adenocarcinoma metastasis. Magn Reson Imaging. 2022;86:37–45. https://doi.org/10.1016/j.mri.2021.11.008
Chen Y, Kim J, Yang S, Wang H, Wu CJ, Sugimoto H, et al. Type I collagen deletion in alphaSMA(+) myofibroblasts augments immune suppression and accelerates progression of pancreatic cancer. Cancer Cell. 2021;39:548–65e6. https://doi.org/10.1016/j.ccell.2021.02.007
Qiang L, Hoffman MT, Ali LR, Castillo JI, Kageler L, Temesgen A, et al. Transforming growth factor-beta blockade in pancreatic cancer enhances sensitivity to combination chemotherapy. Gastroenterology. 2023;165:874–90. e10.
Raymond KN, Pierre VC. Next generation, high relaxivity gadolinium MRI agents. Bioconjug Chem. 2005;16:3–8. https://doi.org/10.1021/bc049817y
Katz MH, Fleming JB, Bhosale P, Varadhachary G, Lee JE, Wolff R, et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer. 2012;118:5749–56. https://doi.org/10.1002/cncr.27636
Vaidya A, Ayat N, Buford M, Wang H, Shankardass A, Zhao Y, et al. Noninvasive assessment and therapeutic monitoring of drug-resistant colorectal cancer by MR molecular imaging of extradomain-B fibronectin. Theranostics. 2020;10:11127–43. https://doi.org/10.7150/thno.47448
Li Y, Han Z, Roelle S, DeSanto A, Sabatelle R, Schur R, et al. Synthesis and assessment of peptide Gd-DOTA conjugates targeting extradomain B fibronectin for magnetic resonance molecular imaging of prostate cancer. Mol Pharm. 2017;14:3906–15. https://doi.org/10.1021/acs.molpharmaceut.7b00619
Zhou Z, Qutaish M, Han Z, Schur RM, Liu Y, Wilson DL, et al. MRI detection of breast cancer micrometastases with a fibronectin-targeting contrast agent. Nat Commun. 2015;6:7984. https://doi.org/10.1038/ncomms8984
Lu ZR, Laney V, Li Y. Targeted contrast agents for magnetic resonance molecular imaging of cancer. Acc Chem Res. 2022;55:2833–47. https://doi.org/10.1021/acs.accounts.2c00346
Han Z, Zhou Z, Shi X, Wang J, Wu X, Sun D, et al. EDB fibronectin specific peptide for prostate cancer targeting. Bioconjug Chem. 2015;26:830–8. https://doi.org/10.1021/acs.bioconjchem.5b00178
Lu G, van den Berg NS, Martin BA, Nishio N, Hart ZP, van Keulen S, et al. Tumour-specific fluorescence-guided surgery for pancreatic cancer using panitumumab-IRDye800CW: a phase 1 single-centre, open-label, single-arm, dose-escalation study. Lancet Gastroenterol Hepatol. 2020;5:753–64. https://doi.org/10.1016/S2468-1253(20)30088-1
Hoogstins CES, Boogerd LSF, Sibinga Mulder BG, Mieog JSD, Swijnenburg RJ, van de Velde CJH, et al. Image-guided surgery in patients with pancreatic cancer: first results of a clinical trial using SGM-101, a novel carcinoembryonic antigen-targeting, near-infrared fluorescent agent. Ann Surg Oncol. 2018;25:3350–7. https://doi.org/10.1245/s10434-018-6655-7
Fu H, Lou K, He H, Wang Y, Mi Y, Li W, et al. A novel PSMA targeted dual-function near-infrared fluorescence and PET probe for the image-guided surgery and detection of prostate cancer. Eur J Nucl Med Mol Imaging. 2023. https://doi.org/10.1007/s00259-023-06492-x
Cheng Z, Ma J, Yin L, Yu L, Yuan Z, Zhang B, et al. Non-invasive molecular imaging for precision diagnosis of metastatic lymph nodes: opportunities from preclinical to clinical applications. Eur J Nucl Med Mol Imaging. 2023;50:1111–33. https://doi.org/10.1007/s00259-022-06056-5
Tirkes T, Yadav D, Conwell DL, Territo PR, Zhao X, Persohn SA, et al. Quantitative MRI of chronic pancreatitis: results from a multi-institutional prospective study, magnetic resonance imaging as a non-invasive method for assessment of pancreatic fibrosis (MINIMAP). Abdom Radiol (NY). 2022;47:3792–805. https://doi.org/10.1007/s00261-022-03654-7
Tirkes T, Shah ZK, Takahashi N, Grajo JR, Chang ST, Venkatesh SK, et al. Reporting standards for chronic pancreatitis by using CT, MRI, and MR cholangiopancreatography: the consortium for the study of chronic pancreatitis, diabetes, and pancreatic cancer. Radiology. 2019;290:207–15. https://doi.org/10.1148/radiol.2018181353
Acknowledgements
This study was funded by the National Natural Science Foundation of China (Grant Nos. 62027901, 82272111, 92159303, 82071896, 81871422, 81871514, and 81227901), Beijing Natural Science Foundation (Grant No. 7212207, 7244524), National High Level Hospital Clinical Research Funding (2022-PUMCH-D-001), and Peking University Third Hospital (BYSYZD2019018 and jyzc2018-02).
Funding
This study was funded by the National Natural Science Foundation of China (Grant Nos. 62027901, 82272111, 92159303, 82071896, 81871422, 81871514, and 81227901), Beijing Natural Science Foundation (Grant No. 7212207, 7244524), National High Level Hospital Clinical Research Funding (2022-PUMCH-D-001), and Peking University Third Hospital (BYSYZD2019018 and jyzc2018-02).
Author information
Authors and Affiliations
Contributions
Conception, design, and supervision were performed by Yang Du, Jie Tian, Zhengyu Jin, and Huadan Xue. Material preparation, data collection, and analyses were performed by Wenjia Zhang, Xiaolong Liang, Xinyu Zhang, Wei Tong, Guangyuan Shi, and Haozhuo Guo. The first draft of the manuscript was written by Wenjia Zhang, Xiaolong Liang, and Xinyu Zhang. The manuscript was edited by Yang Du, Jie Tian, Zhengyu Jin, and Huadan Xue. All authors commented on previous versions of the manuscript. All authors approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
All experimental studies were approved by the Ethics Committee of the Peking Union Medical College Hospital (XHDW-2022-016). The details of the experimental section are available in the supporting information.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, W., Liang, X., Zhang, X. et al. Magnetic-optical dual-modality imaging monitoring chemotherapy efficacy of pancreatic ductal adenocarcinoma with a low-dose fibronectin-targeting Gd-based contrast agent. Eur J Nucl Med Mol Imaging (2024). https://doi.org/10.1007/s00259-024-06617-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00259-024-06617-w