Abstract
Major depressive disorder, a prevalent and severe psychiatric condition, necessitates development of new and fast-acting antidepressants. Genetic suppression of astrocytic inwardly rectifying potassium channel 4.1 (Kir4.1) in the lateral habenula ameliorates depression-like phenotypes in mice. However, Kir4.1 remains an elusive drug target for depression. Here, we discovered a series of Kir4.1 inhibitors through high-throughput screening. Lys05, the most potent one thus far, effectively suppressed native Kir4.1 channels while displaying high selectivity against established targets for rapid-onset antidepressants. Cryogenic-electron microscopy structures combined with electrophysiological characterizations revealed Lys05 directly binds in the central cavity of Kir4.1. Notably, a single dose of Lys05 reversed the Kir4.1-driven depression-like phenotype and exerted rapid-onset (as early as 1 hour) antidepressant actions in multiple canonical depression rodent models with efficacy comparable to that of (S)-ketamine. Overall, we provided a proof of concept that Kir4.1 is a promising target for rapid-onset antidepressant effects.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The structure of Lys05 is accessible from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/) under compound CID 70673566. The cryo-EM density maps and coordinates of RnKir4.1PIP2 and RnKir4.1PIP2-Lys05 have been deposited in the Electron Microscopy Data Bank under accession numbers EMD-35195 and EMD-35196, respectively, and in the RCSB Protein Data Bank under accession codes 8I5M and 8I5N, respectively. Source data are provided with this paper. All other data are available from the corresponding authors upon reasonable request.
References
Malhi, G. S. & Mann, J. J. Depression. Lancet 392, 2299–2312 (2018).
Rush, A. J. et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am. J. Psychiatry 163, 1905–1917 (2006).
Mullard, A. 2019 FDA drug approvals. Nat. Rev. Drug Discov. 19, 79–84 (2020).
Morgan, C. J. A. & Curran, H. V., Independent Scientific Committee on Drugs. Ketamine use: a review. Addict. Abingdon Engl. 107, 27–38 (2012).
Djukic, B., Casper, K. B., Philpot, B. D., Chin, L.-S. & McCarthy, K. D. Conditional knock-out of Kir4.1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation. J. Neurosci. J. Soc. Neurosci. 27, 11354–11365 (2007).
Olsen, M. L. & Sontheimer, H. Functional implications for Kir4.1 channels in glial biology: from K+ buffering to cell differentiation. J. Neurochem. 107, 589–601 (2008).
Neusch, C., Rozengurt, N., Jacobs, R. E., Lester, H. A. & Kofuji, P. Kir4.1 potassium channel subunit is crucial for oligodendrocyte development and in vivo myelination. J. Neurosci. 21, 5429–5438 (2001).
Neusch, C. et al. Lack of the Kir4.1 channel subunit abolishes K+ buffering properties of astrocytes in the ventral respiratory group: impact on extracellular K+ regulation. J. Neurophysiol. 95, 1843–1852 (2006).
Bay, V. & Butt, A. M. Relationship between glial potassium regulation and axon excitability: a role for glial Kir4.1 channels. Glia 60, 651–660 (2012).
Kucheryavykh, Y. V. et al. Downregulation of Kir4.1 inward rectifying potassium channel subunits by RNAi impairs potassium transfer and glutamate uptake by cultured cortical astrocytes. Glia 55, 274–281 (2007).
Chever, O., Djukic, B., McCarthy, K. D. & Amzica, F. Implication of Kir4.1 channel in excess potassium clearance: an in vivo study on anesthetized glial-conditional Kir4.1 knock-out mice. J. Neurosci. 30, 15769–15777 (2010).
Larsen, B. R. et al. Contributions of the Na+/K+-ATPase, NKCC1, and Kir4.1 to hippocampal K+ clearance and volume responses. Glia 62, 608–622 (2014).
Sibille, J., Dao Duc, K., Holcman, D. & Rouach, N. The neuroglial potassium cycle during neurotransmission: role of Kir4.1 channels. PLoS Comput. Biol. 11, e1004137 (2015).
Orkand, R. K., Nicholls, J. G. & Kuffler, S. W. Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J. Neurophysiol. 29, 788–806 (1966).
Nwaobi, S. E., Cuddapah, V. A., Patterson, K. C., Randolph, A. C. & Olsen, M. L. The role of glial-specific Kir4.1 in normal and pathological states of the CNS. Acta Neuropathol. 132, 1–21 (2016).
Tong, X. et al. Astrocyte Kir4.1 ion channel deficits contribute to neuronal dysfunction in Huntington’s disease model mice. Nat. Neurosci. 17, 694–703 (2014).
Kelley, K. W. et al. Kir4.1-dependent astrocyte-fast motor neuron interactions are required for peak strength. Neuron 98, 306–319.e7 (2018).
Hibino, H. et al. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol. Rev. 90, 291–366 (2010).
Cui, Y. et al. Astroglial Kir4.1 in the lateral habenula drives neuronal bursts in depression. Nature 554, 323–327 (2018).
Yang, Y. et al. Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature 554, 317–322 (2018).
Cui, Y., Hu, S. & Hu, H. Lateral habenular burst firing as a target of the rapid antidepressant effects of ketamine. Trends Neurosci. 42, 179–191 (2019).
Ohno, Y., Hibino, H., Lossin, C., Inanobe, A. & Kurachi, Y. Inhibition of astroglial Kir4.1 channels by selective serotonin reuptake inhibitors. Brain Res. 1178, 44–51 (2007).
Su, S. et al. Inhibition of astroglial inwardly rectifying Kir4.1 channels by a tricyclic antidepressant, nortriptyline. J. Pharmacol. Exp. Ther. 320, 573–580 (2007).
Henry, M. E. et al. A comparison of brain and serum pharmacokinetics of R-fluoxetine and racemic fluoxetine: a 19-F MRS study. Neuropsychopharmacology 30, 1576–1583 (2005).
Xiong, Z. et al. Lack of rapid antidepressant effects of Kir4.1 channel inhibitors in a chronic social defeat stress model: comparison with (R)-ketamine. Pharmacol. Biochem. Behav. 176, 57–62 (2019).
Furutani, K., Ohno, Y., Inanobe, A., Hibino, H. & Kurachi, Y. Mutational and in silico analyses for antidepressant block of astroglial inward-rectifier Kir4.1 channel. Mol. Pharmacol. 75, 1287–1295 (2009).
Morán-Zendejas, R. et al. In vitro and in silico characterization of the inhibition of Kir4.1 channels by aminoglycoside antibiotics. Br. J. Pharmacol. 177, 4548–4560 (2020).
Kharade, S. V. et al. Discovery, characterization, and effects on renal fluid and electrolyte excretion of the Kir4.1 potassium channel pore blocker, VU0134992. Mol. Pharmacol. 94, 926–937 (2018).
McClenahan, S. J. et al. VU6036720: the first potent and selective in vitro inhibitor of heteromeric Kir4.1/5.1 inward rectifier potassium channels. Mol. Pharmacol. 101, 357–370 (2022).
McAfee, Q. et al. Autophagy inhibitor Lys05 has single-agent antitumor activity and reproduces the phenotype of a genetic autophagy deficiency. Proc. Natl Acad. Sci. USA 109, 8253–8258 (2012).
Smart, O. S., Goodfellow, J. M. & Wallace, B. A. The pore dimensions of gramicidin A. Biophys. J. 65, 2455–2460 (1993).
Hansen, S. B., Tao, X. & MacKinnon, R. Structural basis of PIP2 activation of the classical inward rectifier K+ channel Kir2.2. Nature 477, 495–498 (2011).
Niu, Y., Tao, X., Touhara, K. K. & MacKinnon, R. Cryo-EM analysis of PIP2 regulation in mammalian GIRK channels. eLife 9, e60552 (2020).
Davis, I. W., Raha, K., Head, M. S. & Baker, D. Blind docking of pharmaceutically relevant compounds using RosettaLigand: blind docking with RosettaLigand. Protein Sci. 18, 1998–2002 (2009).
Qi, Y. et al. CHARMM-GUI MDFF/xMDFF utilizer for molecular dynamics flexible fitting simulations in various environments. J. Phys. Chem. B 121, 3718–3723 (2017).
Wible, B. A., Taglialatela, M., Ficker, E. & Brown, A. M. Gating of inwardly rectifying K+ channels localized to a single negatively charged residue. Nature 371, 246–249 (1994).
Lu, Z. & MacKinnon, R. Electrostatic tuning of Mg2+ affinity in an inward-rectifier K+channel. Nature 371, 243–246 (1994).
Gururajan, A., Reif, A., Cryan, J. F. & Slattery, D. A. The future of rodent models in depression research. Nat. Rev. Neurosci. 20, 686–701 (2019).
Zanos, P. et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533, 481–486 (2016).
Bodnoff, S. R., Suranyi-Cadotte, B., Quirion, R. & Meaney, M. J. A comparison of the effects of diazepam versus several typical and atypical anti-depressant drugs in an animal model of anxiety. Psychopharmacol. 97, 277–279 (1989).
Santarelli, L. et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301, 805–809 (2003).
Ramaker, M. J. & Dulawa, S. C. Identifying fast-onset antidepressants using rodent models. Mol. Psychiatry 22, 656–665 (2017).
Song, C. & Leonard, B. E. The olfactory bulbectomised rat as a model of depression. Neurosci. Biobehav. Rev. 29, 627–647 (2005).
Kryst, J. et al. Efficacy of single and repeated administration of ketamine in unipolar and bipolar depression: a meta-analysis of randomized clinical trials. Pharmacol. Rep. 72, 543–562 (2020).
Griffiths, R. R. et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: a randomized double-blind trial. J. Psychopharmacol. 30, 1181–1197 (2016).
Scholl, U. I. et al. Seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME syndrome) caused by mutations in KCNJ10. Proc. Natl Acad. Sci. USA 106, 5842–5847 (2009).
Bockenhauer, D., Bandulik, S., Tobin, J., Landoure, G. & Thompson, D. Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N. Engl. J. Med. 360, 1960–1970 (2009).
Rebecca, V. W. et al. PPT1 promotes tumor growth and is the molecular target of chloroquine derivatives in cancer. Cancer Discov. 9, 220–229 (2019).
Riggs, L. M. & Gould, T. D. Ketamine and the future of rapid-acting antidepressants. Annu. Rev. Clin. Psychol. 17, 207–231 (2021).
Kaplan, A. L. et al. Bespoke library docking for 5-HT2A receptor agonists with antidepressant activity. Nature 610, 582–591 (2022).
Cameron, L. P. et al. A non-hallucinogenic psychedelic analogue with therapeutic potential. Nature 589, 474–479 (2021).
Cao, D. et al. Structure-based discovery of nonhallucinogenic psychedelic analogs. Science 375, 403–411 (2022).
Sun, N. et al. Design of fast-onset antidepressant by dissociating SERT from nNOS in the DRN. Science 378, 390–398 (2022).
Gourley, S. L. & Taylor, J. R. Recapitulation and reversal of a persistent depression‐like syndrome in rodents. Curr. Protoc. Neurosci. https://doi.org/10.1002/0471142301.ns0932s49 (2009).
Heurteaux, C. et al. Deletion of the background potassium channel TREK-1 results in a depression-resistant phenotype. Nat. Neurosci. 9, 1134–1141 (2006).
Mazella, J. et al. Spadin, a sortilin-derived peptide, targeting rodent TREK-1 channels: a new concept in the antidepressant drug design. PLoS Biol. 8, e1000355 (2010).
Ye, D. et al. TREK1 channel blockade induces an antidepressant-like response synergizing with 5-HT1A receptor signaling. Eur. Neuropsychopharmacol. 25, 2426–2436 (2015).
Friedman, A. K. et al. KCNQ channel openers reverse depressive symptoms via an active resilience mechanism. Nat. Commun. 7, 11671 (2016).
Costi, S. et al. Impact of the KCNQ2/3 channel opener ezogabine on reward circuit activity and clinical symptoms in depression: results from a randomized controlled trial. Am. J. Psychiatry 178, 437–446 (2021).
Lopez, J. P. et al. Ketamine exerts its sustained antidepressant effects via cell-type-specific regulation of Kcnq2. Neuron 110, 2283–2298.e9 (2022).
Li, N. et al. Structure of a pancreatic ATP-sensitive potassium channel. Cell 168, 101–110.e10 (2017).
Ponce-Balbuena, D. et al. Tamoxifen inhibits inward rectifier K+ 2.x family of inward rectifier channels by interfering with phosphatidylinositol 4,5-bisphosphate-channel interactions. J. Pharmacol. Exp. Ther. 331, 563–573 (2009).
Ohno, Y., Kinboshi, M. & Shimizu, S. Inwardly rectifying potassium channel Kir4.1 as a novel modulator of BDNF expression in astrocytes. Int. J. Mol. Sci. 19, E3313 (2018).
Song, Z. et al. Astrocytic Kir4.1 regulates NMDAR/calpain signaling axis in lipopolysaccharide-induced depression-like behaviors in mice. Toxicol. Appl. Pharmacol. 429, 115711 (2021).
Pearson, W. L., Skatchkov, S. N., Eaton, M. J. & Nichols, C. G. C-terminal determinants of Kir4.2 channel expression. J. Membr. Biol. 213, 187–193 (2006).
Zhang, Y.-M. et al. Discovery of HN37 as a potent and chemically stable antiepileptic drug candidate. J. Med. Chem. 64, 5816–5837 (2021).
Wu, X. et al. ML365 inhibits TWIK2 channel to block ATP-induced NLRP3 inflammasome. Acta Pharmacol. Sin. 43, 992–1000 (2022).
Jiang, L. I. et al. Use of a cAMP BRET sensor to characterize a novel regulation of cAMP by the sphingosine 1-phosphate/G13 pathway. J. Biol. Chem. 282, 10576–10584 (2007).
Schildge, S., Bohrer, C., Beck, K. & Schachtrup, C. Isolation and culture of mouse cortical astrocytes. J. Vis. Exp. https://doi.org/10.3791/50079 (2013).
de Almeida, R. F., Pocharski, C. B., Rodrigues, A. L. S., Elisabetsky, E. & Souza, D. O. Guanosine fast onset antidepressant-like effects in the olfactory bulbectomy mice model. Sci. Rep. 10, 8429 (2020).
Mendez-David, I. et al. Rapid anxiolytic effects of a 5-HT4 receptor agonist are mediated by a neurogenesis-independent mechanism. Neuropsychopharmacology 39, 1366–1378 (2014).
Wang, H. et al. Norbin ablation results in defective adult hippocampal neurogenesis and depressive-like behavior in mice. Proc. Natl Acad. Sci. USA 112, 9745–9750 (2015).
Yalcin, I., Aksu, F. & Belzung, C. Effects of desipramine and tramadol in a chronic mild stress model in mice are altered by yohimbine but not by pindolol. Eur. J. Pharmacol. 514, 165–174 (2005).
Octeau, J. C., Faas, G., Mody, I. & Khakh, B. S. Making, testing, and using potassium ion selective microelectrodes in tissue slices of adult brain. J. Vis. Exp. https://doi.org/10.3791/57511 (2018).
Nicholson, C. Ion-selective microelectrodes and diffusion measurements as tools to explore the brain cell microenvironment. J. Neurosci. Methods 48, 199–213 (1993).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Zhang, K. Gctf: real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016).
The PyMOL Molecular Graphics System v.1.8 (Schrödinger LLC, 2015).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D. Biol. Crystallogr. 66, 486–501 (2010).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D. Biol. Crystallogr. 66, 213–221 (2010).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D. Biol. Crystallogr. 66, 12–21 (2010).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Vanommeslaeghe, K. et al. CHARMM general force field: a force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. https://doi.org/10.1002/jcc.21367 (2009).
Huang, J. et al. CHARMM36m: an improved force field for folded and intrinsically disordered proteins. Nat. Methods 14, 71–73 (2017).
Phillips, J. C. et al. Scalable molecular dynamics with NAMD. J. Comput. Chem. 26, 1781–1802 (2005).
Acknowledgements
We thank Y. Li and F. Guo for assistance with in vivo electrophysiology. We thank C. Xie for advice on renal metabolism experiments. Z.G. is supported by the National Science Fund for Distinguished Young Scholars (grant no. 81825021), the National Natural Science Foundation of China (grant no. 92169202), the National Science and Technology Innovation 2030 Major Program (grant no. 2021ZD0200900) and the Shanghai Municipal Science and Technology Major Project (grant no. 2018SHZDZX05). Y. Zheng is supported by the Youth Innovation Promotion Association of the Chinese Academy of Sciences (grant no. 2020284) and the Lingang Laboratory (grant nos. LG202103-01-06, LG202103-01-05). J.G. is supported by MOE Frontier Science Center for Brain Science & Brain-Machine Integration, Zhejiang University.
Author information
Authors and Affiliations
Contributions
X.Z., Y. Zheng and J.H. performed most electrophysiological, molecular biological experiments. C.Z. and J.G. performed the cryo-EM and image analysis. Q.C. conducted the ITC assay. H.X., X.Z., L.Z., P.W. and Y.G. performed behavioral experiments. Y.X. and T.L. synthesized the compound under the supervision of J. Li. C.X., Y.C. and Y.L. performed functional assays on 5-HT receptors and metabotropic glutamate receptors under the supervision of J. Liu. X.X. performed and supervised functional assays of opioid receptors. Y. Zeng and F.T. conducted electrophysiological studies on glutamate and GABAA receptors. X.Z., Y. Zheng and H.X. analyzed cellular and behavioral data. H.H. and Y.Y. participated in the behavioral data analysis. Z.G., X.Z., Y. Zheng and J.G. designed the research and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
H.H. and Y.Y. are named on a patent for use of potassium channel inhibitors for treating depression (US20200149051A1). Z.G., X.Z., Y. Zheng, H.X., P.W., L.Z. and Y.G. are inventors on a patent pending for the use of Lys05 to treat depression or conditions related to Kir4.1 inhibition. All other authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks Jerod Denton, Todd Gould and Zhiguang Yuchi for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Nine drug candidates targeting Kir4.1 channel discovered in fluorescence-based drug screening and validated by whole-cell patch-clamp recording.
a, The overall results of the primary screening with nine drug candidates highlighted in colored dots. b, Bar graph showing the effects of Lys05 and eight other small-molecules on Kir4.1 at 1 and 10 μM in the whole-cell patch-clamp recording (n = 4 cells for each bar). c, Chemical structures of the nine candidates. Data are means ± s.e.m.
Extended Data Fig. 2 The pharmacological profile of Lys05.
a, Concentration-response curves for Lys05 inhibition of recombinant Kir channels (Kir1.1, n = 6; Kir2.1, n = 4; Kir2.2, n = 5; Kir2.3, n = 5; Kir3.2, n = 4; Kir4.1, n = 8; Kir4.2 Y355F, n = 5; and Kir4.1/5.1, n = 11). Data are means ± s.e.m. b, Concentration-response curves of Lys05 and respective reference compound against antidepressant-relevant channels, GPCRs and monoaminergic transporters. For GPCRs and transporters, graphs show one representative result performed in triplicate or quadruplicate of 3 independent experiments. For channels, graphs show average results of all cells measured (GluN1/GluN2A, n = 12; GluN1/GluN2B, n = 10; α1β3γ2 GABAA, n = 7; α5β3γ2 GABAA, n = 4; and AMPA receptors, n = 5). Data are means ± s.e.m.
Extended Data Fig. 3 Structure determination of RnKir4.1PIP2.
a, Size-exclusion chromatography of RnKir4.1PIP2 on superose 6 (GE Healthcare). b, SDS-PAGE analysis of the RnKir4.1PIP2 sample (n = 3 independent experiments). c, Representative cryo-EM micrograph of RnKir4.1PIP2 (n = 2915 micrographs). d, Flowchart of image processing for RnKir4.1PIP2 particles. e, Angular distribution plot of particles included in the final C4-symmetric 3D reconstruction of RnKir4.1PIP2. f, The Gold standard Fourier Shell Correlation (FSC) curves of the final 3D reconstructions of RnKir4.1PIP2. g, The 3D reconstruction of RnKir4.1PIP2 colored by local resolution in Å. h, The FSC curves for cross-validation between the map and the model of RnKir4.1PIP2. i, Sample maps at 2 transmembrane helices of RnKir4.1PIP2 at the contour level of 5 σ. j, No density map in the central cavity of RnKir4.1PIP2 at the level of 0.01 in UCSF chimera. k, Density of PIP2 and its surrounding residues in RnKir4.1PIP2 at the contour level of 5 σ.
Extended Data Fig. 4 Structure determination of RnKir4.1PIP2-Lys05.
a, Representative cryo-EM micrograph of RnKir4.1PIP2-Lys05 (n = 2700 micrographs). b, Flowchart of image processing for RnKir4.1PIP2-Lys05 particles. The final 3D reconstructions were performed with C4 and C1 symmetries, respectively. c, Angular distribution plot of particles included in the final C4-symmetric 3D reconstruction of RnKir4.1PIP2-Lys05. d, The Gold standard Fourier Shell Correlation (FSC) curves of the final 3D reconstructions of RnKir4.1PIP2-Lys05. e, The 3D reconstruction of RnKir4.1PIP2-Lys05 colored by local resolution in Å. f, The FSC curves for cross-validation between the map and the model of RnKir4.1PIP2-Lys05. g, Sample maps at 2 transmembrane helices of RnKir4.1PIP2-Lys05 at the contour level of 5 σ. h, Structural comparison of RnKir4.1PIP2 and RnKir4.1PIP2-Lys05 when the entire channels are aligned (left) and a zoom-in view of residues located on the surface of the central pocket (right). The RMSD over 310 Cα atoms within one subunit between two structures is 0.44 Å. i, In the C1 model of RnKir4.1PIP2-Lys05, the diagonal atom-to-atom distance between Cα of Ala243 located in the entrances of cytosolic pocket are 26.40 Å and 27.00 Å, respectively. j, Superimposition of the docked Lys05 and the density in the central pocket of the Kir4.1PIP2-Lys05 map. k, The ITC showed that one RnKir4.1 subunit can bind ~0.5 Lys05 molecule.
Extended Data Fig. 5 Residues of E158, I159 and T128 underlie the blockade of Kir4.1 by Lys05.
a, Representative current traces of Kir4.1 wild-type and mutant channels before and after perfusion of Lys05 at indicated concentrations in the external 30 mM K+. b, Sequence alignment of Kir4.1 and Lys05-insensitive Kir1.1 (left). The mutant channels, Kir1.1 N171E (n = 3 cells) and S172I (n = 3 cells), gained inhibition by 10 μM Lys05 compared to the wild-type channel (n = 4 cells, right). One-way ANOVA with Tukey’s post hoc test. Data are means ± s.e.m.
Extended Data Fig. 6 Lys05 decreased native Kir4.1 currents in primary cortical astrocytes.
a, Representative whole-cell current traces from cultured astrocytes transfected with control siRNA (top, n = 9 astrocytes) or siRNA targeting Kcnj10 (bottom, n = 8 astrocytes) in bath solution, with addition of 10 μM Lys05 and washout. The summary graph is shown in Fig. 3e-f. The protocols are as indicated in the insets. b, Representative current traces (top and middle) and bar graph (bottom) showing the inhibitory effects of 10 μM Lys05 on native Kir4.1 currents with a ramp stimulus in astrocytes treated with control siRNA (n = 12 cells) or siRNA targeting Kcnj10 (n = 7 cells). c, Representative RMP traces from control (top, n = 7 astrocytes) or Kir4.1-silenced astrocytes (bottom, n = 11 astrocytes) in response to bath application of 100 μM BaCl2. d, Comparison of the effects of 100 μM BaCl2 on astrocytic RMPs between control and Kir4.1-silenced astrocytes (n, indicated in brackets). Perfusion of 100 μM BaCl2 induced depolarization in control astrocytes but not Kir4.1-silenced astrocytes. Two-tailed Student’s t-test. Data are means ± s.e.m.
Extended Data Fig. 7 The antidepressant effects of Lys05 in mice with viral injection into the LHb.
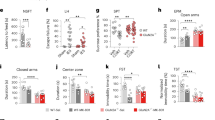
a, Functional characterizations of AAV constructs expressing eGFP-Kir4.1 or eGFP alone under a gfaABC1D promoter in astrocytes (n = 3 biological samples per group). Two-tailed Student’s t-test. The primary cultured astrocytes (DIV 23) were infected with the viruses and collected for expressional analysis in 72 h. b, Left, the effects of expression of AAV constructs in the LHb and intraperitoneal administration of Lys05 on NSF test result (n = 11, 10, 8, 6 mice, respectively; Kaplan–Meier survival analysis with Mantel-Cox test). Right, food consumption in home cage (n, indicated in brackets; two-tailed Student’s t-test for Control AAV-Veh versus Kir4.1 AAV-Veh group and one-way ANOVA for Kir4.1 AAV-Veh versus Kir4.1 AAV-Drug groups). c, Effects of Lys05 on immobility time (left; n, indicated in brackets) and immobility episodes (right; n, indicated in brackets) in the FST in mice injected with control viruses. One-way ANOVA. d, Effects of Lys05 on sucrose intake ratio in the SPT in mice injected with control viruses (n, indicated in brackets). One-way ANOVA. Data are means ± s.e.m.
Extended Data Fig. 8 In vivo effects of Lys05 relative to (S)-ketamine in depression models.
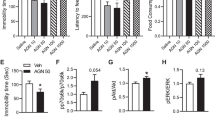
a, Schematic overview (top) and the antidepressant-like effects of three repeated injections of Lys05 in NSF test (bottom, n = 9 mice per group). Kaplan–Meier survival analysis with Mantel-Cox test. b, Schematics overview (top) and the effects of acute Lys05 or ketamine treatment (i.p.) at 1 h after administration on OFT results (n, indicated in brackets). Two-tailed Student’s t-test for H2O-Veh versus CORT-Veh group and one-way ANOVA with Dunnett’s post hoc test for CORT-Veh versus CORT-Drug groups. The corresponding results of TST and SPT were shown in Fig.5. c, Verification of removed olfactory bulbs in OBX mice. Mice with complete olfactory bulbs removal and without frontal cortex injuries were used in behavioral analysis. d, Schematics overview (top) and the effects of an acute administration of Lys05 (10 and 30 mg/kg), imipramine (30 mg/kg) and (S)-ketamine (3 and 10 mg/kg) on the anxiety-like behavior in OBX mice (n, indicated in brackets). Two-way ANOVA with Bonferroni post hoc test. e, Schematic overview (top) and the effects of five repeated injections of Lys05 as well as imipramine on total distance travelled (left bottom) and percentage of distance travelled in center (right bottom) (n, indicated in brackets). Two-way ANOVA with Bonferroni post hoc test. Data are means ± s.e.m.
Extended Data Fig. 9 Photoaffinity pulldown experiments, extracellular K+ measurement and characterization of the I159A mutation in mice.
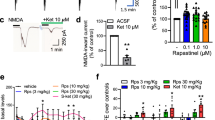
a, Concentration-response curves of L-probe (maroon, n = 6 cells) and Lys05 (black, n = 8 cells) at recombinant Kir4.1 channels. The inset shows the chemical structure of L-probe. b, Representative immunoblots showing Kir4.1 protein levels pulled down from brain lysates treated with either L-probe or L-probe plus Lys05 competition at the indicated concentrations (left) and quantification of western blot results from n = 2 independent experiments (right). c, Schematics showing in vivo extracellular K+ measurement in the LHb region using K+-selective microelectrodes following systemic administration of 30 mg/kg Lys05. d, The calibration curve of the K+-selective microelectrodes (n = 4). e, Left, changes of [K+]o during systemic Lys05 administration (n = 3, 4 mice). Right, representative traces in the [K+]o recordings following i.p. injections of Lys05 (blue) or the solvent (grey). f, Unaltered Kir4.1 expression in the habenula and hippocampus in wild-type and I159A mutant mice (n = 4, 4, 4 biological samples for habenula; n = 7, 6, 5 biological samples for hippocampus). One-way ANOVA. g, The RMPs in primary cortical astrocytes from wild-type and I159A mutant mice and the sensitivities to 10 μM Lys05 treatment (n, indicated in brackets). Two-way ANOVA with Bonferroni post hoc test. Data are means ± s.e.m.
Extended Data Fig. 10 Evaluation of the safety profile of Lys05 in vivo.
a, Schematics showing the experimental design. Mice were treated with a single dose (10 or 30 mg/kg, 0.5 h ahead) or multiple doses (30 mg/kg per dose, once daily for 5 days) of Lys05 and assigned to behavioral assessments. b, Seizure susceptibility of mice treated with a single injection (n = 6-8 mice per stimulus intensity per group) of Lys05 at the indicated doses in the 6-Hz seizure test. Left, CS50 (Vehicle) = 18.1 (16.8-19.4) mA; CS50 (10 mg/kg Lys05) = 18.0 (17.1-18.8) mA; Right, CS50 (Vehicle) = 16.9 (15.5-18.6) mA; CS50 (30 mg/kg Lys05) = 19.1 (18.3-19.8) mA. c, Mice treated with multiple injections in the same assay (n = 4-7 mice per stimulus intensity per group). CS50 (Vehicle)= 15.3 (11.5-20.5) mA; CS50 (Lys05) = 15.8 (13.1-19.0) mA. d, Seizure susceptibility of mice treated with a single dose (n = 6-8 mice per stimulus intensity per group) of Lys05 as indicated in the MES test. Left, CS50 (Vehicle) = 0.68 (0.62-0.74) mA; CS50 (10 mg/kg Lys05) = 0.67 (0.65-0.69) mA. Right, CS50 (Vehicle) = 0.69 (0.60-0.76) mA; CS50 (30 mg/kg Lys05) = 0.75 (0.60-0.80) mA. e, Mice treated with multiple injections in the same assay (n = 4-7 mice per stimulus intensity per group). CS50 (Vehicle) = 0.65 (0.59-0.70) mA; CS50 (Lys05) = 0.67 (0.64-0.71) mA. f, Effects of Lys05 treatment at indicated doses on motor coordination in the rotarod test (n = 10-25 mice per group). Data are percentage of mice with seizure manifestations in b-e and N/T values illustrated in f.
Supplementary information
Supplementary Information
Supplementary Tables 1–4, Figs. 1–7 and Notes 1 and 2.
Supplementary Data 1
Source Data for supplementary figures.
Source data
Source Data Fig. 3 and Extended Data Figs. 7 and 9
Unprocessed blots for Fig. 3b and Extended Data Figs. 7a and 9b,f.
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, X., Zhao, C., Xu, H. et al. Pharmacological inhibition of Kir4.1 evokes rapid-onset antidepressant responses. Nat Chem Biol (2024). https://doi.org/10.1038/s41589-024-01555-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41589-024-01555-y
This article is cited by
-
Dam antidepressants
Nature Chemical Biology (2024)