Abstract
Objective
To assess the impact of active surveillance and decolonization strategies on methicillin-resistant Staphylococcus aureus (MRSA) infection rates in a NICU.
Study design
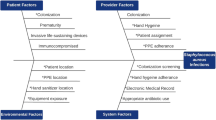
MRSA infection rates were compared before (2014–2016) and during (2017–2022) an active surveillance program. Eligible infants were decolonized with chlorohexidine gluconate (CHG) bathing and/or topical mupirocin. Successful decolonization and rates of recolonization were assessed.
Results
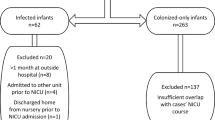
Fifty-two (0.57%) of 9 100 hospitalized infants had invasive MRSA infections from 2014 to 2022; infection rates declined non-significantly. During the 6-year surveillance program, the risk of infection was 16.9-times [CI95 8.4, 34.1] higher in colonized infants than uncolonized infants. Those colonized with mupirocin-susceptible MRSA were more likely successfully decolonized (aOR 9.7 [CI95 4.2, 22.5]). Of 57 infants successfully decolonized who remained hospitalized, 34 (60%) became recolonized.
Conclusions
MRSA infection rates did not significantly decline in association with an active surveillance and decolonization program. Alternatives to mupirocin and CHG are needed to facilitate decolonization.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to privacy concerns, but could be made available from the corresponding author on reasonable request and with the permission of the Columbia University Irving Medical Center IRB.
References
Centers for Disease Control and Prevention. Recommendations for Prevention and Control of Infections in NICU patients: S. aureus. [Internet]. cdc.gov; 2020. Accessed February 2023. https://www.cdc.gov/infectioncontrol/guidelines/nicu-saureus/introduction.html
Nelson MU, Gallagher PG. Methicillin-resistant Staphylococcus aureus in the neonatal intensive care unit. Semin Perinatol. 2012;36:424–30.
Karchmer TB, Durbin LJ, Simonton BM, Farr BM. Cost-effectiveness of active surveillance cultures and contact/droplet precautions for control of methicillin-resistant Staphylococcus aureus. J Hosp Infect. 2002;51:126–32.
Schuetz CR, Hogan PG, Reich PJ, Halili S, Wiseman HE, Boyle MG, et al. Factors associated with progression to infection in methicillin-resistant Staphylococcus aureus-colonized, critically ill neonates. J Perinatol. 2021;41:1285–92.
Maraqa NF, Aigbivbalu L, Masnita-Iusan C, Wludyka P, Shareef Z, Bailey C, et al. Prevalence of and risk factors for methicillin-resistant Staphylococcus aureus colonization and infection among infants at a level III neonatal intensive care unit. Am J Infect Control. 2011;39:35–41.
Zervou FN, Zacharioudakis IM, Ziakas PD, Mylonakis E. MRSA colonization and risk of infection in the neonatal and pediatric ICU: a meta-analysis. Pediatrics. 2014;133:e1015–1023.
Huang YC, Chou YH, Su LH, Lien RI, Lin TY. Methicillin-resistant Staphylococcus aureus colonization and its association with infection among infants hospitalized in neonatal intensive care units. Pediatrics. 2006;118:469–74.
Sakaki H, Nishioka M, Kanda K, Takahashi Y. An investigation of the risk factors for infection with methicillin-resistant Staphylococcus aureus among patients in a neonatal intensive care unit. Am J Infect Control. 2009;37:580–6.
Pierce R, Lessler J, Popoola VO, Milstone AM. Methicillin-resistant Staphylococcus aureus (MRSA) acquisition risk in an endemic neonatal intensive care unit with an active surveillance culture and decolonization programme. J Hosp Infect. 2017;95:91–97.
Kotloff KL, Shirley DT, Creech BC, Frey SE, Harrison CJ, Staat M, et al. Mupirocin for Staphylococcus aureus decolonization of infants in neonatal intensive care units. Pediatrics. 2019;143:e20181565.
Goldstein ND, Jenness SM, Tuttle D, Power M, Paul DA, Eppes SC. Evaluating a neonatal intensive care unit MRSA surveillance programme using agent-based network modelling. J Hosp Infect. 2018;100:337–43.
Geva A, Wright SB, Baldini LM, Smallcomb JA, Safran C, Gray JE. Spread of methicillin-resistant Staphylococcus aureus in a large tertiary NICU: Network analysis. Pediatrics. 2011;128:e1173–1180.
Nelson MU, Shaw J, Gross SJ. Randomized placebo-controlled trial of topical mupirocin to reduce Staphylococcus aureus colonization in infants in the neonatal intensive care unit. J Pediatr. 2021;236:70–77.
Milstone AM, Budd A, Shepard JW, Ross T, Aucott S, Carroll KC. Role of decolonization in a comprehensive strategy to reduce methicillin-resistant Staphylococcus aureus infections in the neonatal intensive care unit: an observational cohort. Infect Control Hosp Epidemiol. 2010;31:558–60.
Bozzella MJ, Soghier L, Harris T, Zell L, Short BL, Song X. Impact of decolonization on methicillin-resistant Staphylococcus aureus transmission and infection in a neonatal intensive care unit. Infect Control Hosp Epidemiol. 2019;40:1123–7.
Popoola VO, Budd A, Wittig SM, Ross T, Aucott SW, Perl TM, et al. Methicillin-resistant Staphylococcus aureus transmission and infections in a neonatal intensive care unit despite active surveillance cultures and decolonization: challenges for infection prevention. Infect Control Hosp Epidemiol. 2014;35:412–8.
Grohs E, Hill-Ricciuti A, Kelly N, Messina M, Green DA, Geng W, et al. Spa typing of Staphylococcus aureus in a neonatal intensive care unit during routine surveillance. J Pediatr Infect Dis Soc. 2021;10:766–73.
Saiman L, Acker KP, Dumitru D, Messina M, Johnson C, Zachariah P, et al. Infection prevention and control for labor and delivery, well baby nurseries, and neonatal intensive care units. Semin Perinatol. 2020;44:151320.
Macnow T, O’Toole D, DeLaMora P, Murray M, Rivera K, Whittier S, et al. Utility of surveillance cultures for antimicrobial resistant organisms in infants transferred to the NICU. Pediatr Infect Dis J. 2013;32:e443–e450.
Carey AJ, Della-Latta P, Huard R, Wu F, Graham PL, Carp D, et al. Changes in the molecular epidemiology of methicillin-resistant Staphylococcus aureus in a neonatal intensive care unit. Infect Control Hosp Epidemiol. 2010;31:613–9.
Swenson JM, Wong B, Simor AE, Thomson RB, Ferraro MJ, Hardy DJ, et al. Multicenter study to determine disk diffusion and broth microdilution criteria for prediction of high- and low-level mupirocin resistance in Staphylococcus aureus. J Clin Microbiol. 2010;48:2469–75.
National Healthcare Safety Network. CDC/NHSN Surveillance Definitions for Specific Types of Infections [Internet]. cdc.gov; 2023. Accessed February 2023. Available from: https://www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf
National Healthcare Safety Network. Surgical Site Infection Event (SSI) [Internet]. cdc.gov; 2023. Accessed February 2023. Available from: https://www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf
National Healthcare Safety Network. Urinary Tract Infection (Catheter-Associated Urinary Tract Infection [CAUTI] and Non-Catheter-Associated Urinary Tract Infection [UTI]) Events. Accessed February 2023. Available from: https://www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf
National Healthcare Safety Network. Pneumonia (Ventilator-associated [VAP] and non-ventilator-associated Pneumonia [PNEU]) Event. Accessed February 2023. Available from: https://www.cdc.gov/nhsn/pdfs/pscmanual/6pscvapcurrent.pdf
Baker MA, Sands KE, Huang SS, Kleinman K, Septimus EJ, Varma N, et al. The impact of COVID-19 on healthcare-associated infections. Clin Infect Dis. 2022;74:1748–54.
Lastinger LM, Alvarez CR, Kofman A, Konnor RY, Kuhar DT, Nkwata A, et al. Continued increases in the incidence of healthcare-associated infection (HAI) during the second year of the coronavirus disease 2019 (COVID-19) pandemic. Infect Control Hosp Epidemiol. 2023;44:997–1001.
Balamohan A, Beachy J, Kohn N, Rubin LG. The effect of routine surveillance and decolonization on the rate of Staphylococcus aureus infections in a level IV neonatal intensive care unit. J Perinatol. 2020;40:1644–51.
Jernigan JA, Titus MG, Groschel DHM, Getchell-White SI, Farr BM. Effectiveness of contact isolation during a hospital outbreak of methicillin-resistant Staphylococcus aureus. Am J Epidemiol. 1996;143:496–504.
Hetem DJ, Bonten MJ. Clinical relevance of mupirocin resistance in Staphylococcus aureus. J Hosp Infect. 2013;85:249–56.
Kamity R, Hanna Z. Chlorhexidine baths in preterm infants - are we there yet? J Perinatol. 2019;39:1014–5.
Bhalla A, Aron DC, Donskey CJ. Staphylococcus aureus intestinal colonization is associated with increased frequency of S. aureus on skin of hospitalized patients. BMC Infect Dis. 2007;7:105.
Ringberg H, Petersson AC, Walder M, Johansson PJH. The throat: an important site for MRSA colonization. Scand J Infect Dis. 2006;38:888–93.
Reeves L, Barton L, Nash M, Williams J, Guimera D, Simmons B, et al. Effectiveness of an alcohol-based nasal antiseptic in reducing MRSA bacteremia in an adult intensive care population. Infect Control Hosp Epidemiol. 2020;41:s206.
Steed LL, Costello J, Lohia S, Jones T, Spannhake EW, Nguyen S. Reduction of nasal Staphylococcus aureus carriage in health care professionals by treatment with a nonantibiotic, alcohol-based nasal antiseptic. Am J Infect Control. 2014;42:841–6.
Poovelikunnel T, Gethin G, Humphreys H. Mupirocin resistance: clinical implications and potential alternatives for the eradication of MRSA. J Antimicrob Chemother. 2015;7:2681–92.
Akinboyo IC, Voskertchian A, Gorfu G, Betz J, Ross T, Carroll KC, et al. Epidemiology and risk factors for recurrent Staphylococcus aureus colonization following active surveillance and decolonization in the NICU. Infect Control Hosp Epidemiol. 2019;39:1334–9.
Advani SD, Murray TS, Murdzek CM, Aniskiewicz MJ, Bizzarro MJ. Shifting focus toward healthcare-associated bloodstream infections: The need for neonatal intensive care unit–specific NHSN definitions. Infect Control Hosp Epidemiol. 2019;41:181–6.
Paul AA, Gentzler E, Solowey K, Manickam S, Frantzis I, Alba L, et al. Epidemiology, risk factors, and applicability of CDC definitions for healthcare-associated bloodstream infections at a level IV neonatal ICU. J Perinatol. 2023;43:1152–7.
Akinboyo IC, Zangwill KM, Berg WM, Cantey JB, Huizinga B, Milstone AM. SHEA neonatal intensive care unit (NICU) white paper series: practical approaches to Staphylococcus aureus disease prevention. Infect Control Hosp Epidemiol. 2020;41:1251–7.
Acknowledgements
We thank the neonatal intensive care unit teams for obtaining the surveillance swabs and implementing the decolonization strategies.
Author information
Authors and Affiliations
Contributions
SG conducted primary data collection and analysis and wrote the first draft of the manuscript. CO conducted primary data collection and analysis and critically reviewed the manuscript. MM supervised collection of surveillance swabs, conducted primary data collection for colonization results and infections, and critically reviewed the manuscript. DAG supervised surveillance culture processing and interpretation and critically reviewed the manuscript. FW assisted with surveillance cultures, performed and analyzed pulsed field gel electrophoresis and critically reviewed the manuscript. AP assisted in data interpretation and critically reviewed the manuscript. BM assisted with the data analysis plan and critically reviewed the manuscript. AHR assisted with the data analysis plan and critically reviewed the manuscript. RS supervised implementation of decolonization efforts and critically reviewed the manuscript. LS conceived the study, reviewed the data collection and analysis plan, and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gollerkeri, S., Oliver, C., Maria, M. et al. Impact of active surveillance and decolonization strategies for methicillin-resistant Staphylococcus aureus in a neonatal intensive care unit. J Perinatol (2024). https://doi.org/10.1038/s41372-024-01902-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41372-024-01902-w