Abstract
Purpose
The aim of this study was to investigate the biodistribution of (super-)selective trans-arterial radioembolization (TARE) with holmium-166 microspheres (166Ho-MS), when administered as adjuvant therapy after RFA of HCC 2–5 cm. The objective was to establish a treatment volume absorbed dose that results in an absorbed dose of ≥ 120 Gy on the hyperemic zone around the ablation necrosis (i.e., target volume).
Methods
In this multicenter, prospective dose-escalation study in BCLC early stage HCC patients with lesions 2–5 cm, RFA was followed by (super-)selective infusion of 166Ho-MS on day 5–10 after RFA. Dose distribution within the treatment volume was based on SPECT-CT. Cohorts of up to 10 patients were treated with an incremental dose (60 Gy, 90 Gy, 120 Gy) of 166Ho-MS to the treatment volume. The primary endpoint was to obtain a target volume dose of ≥ 120 Gy in 9/10 patients within a cohort.
Results
Twelve patients were treated (male 10; median age, 66.5 years (IQR, [64.3–71.7])) with a median tumor diameter of 2.7 cm (IQR, [2.1–4.0]). At a treatment volume absorbed dose of 90 Gy, the primary endpoint was met with a median absorbed target volume dose of 138 Gy (IQR, [127–145]). No local recurrences were found within 1-year follow-up.
Conclusion
Adjuvant (super-)selective infusion of 166Ho-MS after RFA for the treatment of HCC can be administered safely at a dose of 90 Gy to the treatment volume while reaching a dose of ≥ 120 Gy to the target volume and may be a favorable adjuvant therapy for HCC lesions 2–5 cm.
Trial registration
Clinicaltrials.gov NCT03437382. (registered: 19-02-2018)
Similar content being viewed by others
Introduction
In the management of hepatocellular carcinoma (HCC), thermal ablation (TA) has become the preferred curative treatment for lesions up to 2 cm, owing to its equal effectiveness and lower complication rate compared to surgical techniques [1, 2]. For larger tumors, surgical resection is generally regarded as the recommended treatment, provided that liver function is preserved [1,2,3,4,5,6,7]. Nevertheless, most patients are not eligible for surgery due to the presence of underlying liver cirrhosis induced portal hypertension, impaired liver function, other comorbidity, and/or an unfavorable tumor location [1]. As a result, these patients are often treated with TA or trans-arterial therapies, such as trans-arterial chemoembolization (TACE) or trans-arterial radioembolization (TARE) [1, 2].
The risk of developing local recurrence after TA is generally considered to be higher than after surgical resection, especially for lesions > 3 cm [5, 6, 8]. Local recurrences are mainly caused by (a) insufficient heat propagation during thermal ablation, (b) heat sink effect in case of tumors with a bordering intrahepatic vessel, or (c) the presence of viable satellite nodules. Most recurrences are found in the periphery of, or in close proximity to the treated tumor [9].
In order to reduce local recurrence rates after TA of larger lesions (> 3 cm), the combined treatment of TA with TACE has been studied previously. Although the combined treatment may improve survival as compared to TA alone, superiority over surgical treatment has not been proven [10, 11]. Preclinical studies identified potential benefits of combined radiofrequency ablation (RFA) and radiation-based therapies [12,13,14,15]. However, the liver has a low tolerability to external beam radiation therapy [16, 17]. TARE provides a selective way of delivering high doses of radiation therapy to a tumor while saving healthy parenchyma [18, 19] and may work synergistically with RFA when the two therapies are combined.
Since RFA induces hyperemia around the ablation zone [20], this reactive viable liver parenchyma corresponds to the volume where residual tumor cells or satellite nodules are most likely to reside, if present [9]. We hypothesized that this hyperemic effect can be used to deliver a high absorbed dose of holmium-166 microspheres (166Ho-MS) to the tissue directly bordering the ablated tissue with the aim of decreasing chances of developing local recurrences. Early studies on TARE dosimetry reported on higher response rates in patients who received ≥ 120 Gy of yttrium-90 (90Y) monotherapy on their nonresectable HCC, compared to patients who received a lower absorbed dose [21]. The primary objective of this prospective study was to find the treatment volume absorbed dose of 166Ho-MS that yields an absorbed dose of ≥ 120 Gy to the hyperemic zone (target volume). Secondary objectives were to investigate safety and efficacy of this adjuvant therapy.
Materials and methods
Design
The HORA EST HCC study (NCT03437382) was a multicenter (3 tertiary referral centers for HCC), open-label, non-randomized phase Ib dose-escalation study to the use of adjuvant TARE after RFA in patients with Barcelona Clinic for Liver Cancer (BCLC) early stage HCC (A) lesions of 2–5 cm [2]. The study protocol was approved by the local Medical Ethics Committee and was performed in accordance with good clinical practice and the Declaration of Helsinki. All participants provided written informed consent. The full study protocol has been published earlier, in accordance with good research practice [22].
Patients
Eligible patients were those with BCLC early stage HCC (A) with a solitary lesion of 2–5 cm or with up to 3 lesions of ≤ 3 cm and at least one lesion > 2 cm, in whom surgical resection was not the treatment of first choice upon decision by the multidisciplinary tumor board. Main inclusion criteria were age of ≥ 18 years old, Child-Pugh (CP) A or B ≤ 7, an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, an estimated TARE treatment volume ≤ 50% of the total liver volume, no prior hemi-hepatectomy or radiation therapy, and a creatinine clearance rate ≥ 30 mL/min. A list of all in- and exclusion criteria can be found in Table 1.
Study procedures
A schematic overview of the study procedures can be found in Fig. 1. On the first day of treatment, ultrasound or CT guided RFA was performed under general anesthesia using 3 × 3 or 3 × 4 cm exposed tip multi-electrode Cool-tip™ RFA system, electrodes and switching controller (Medtronic Inc, Dublin, Ireland). Immediately after RFA, a contrast enhanced computed tomography (CECT) scan was performed on a 64-slice Aquilion CT-scanner (Canon, Tochigi, Japan) and an additional ablation was acquired in the same session in case residual viable tumor tissue was identified on this scan.
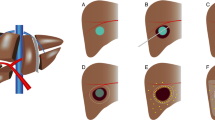
Schematic drawings of the study procedure. A HCC lesion of 2–5 cm. B Thermal ablation of HCC lesion. C Potential sites of local recurrences due to impaired heat propagation, heat-sink effect, or satellite nodules. D Target volume for adjuvant TARE. E Deposition of 166Ho-MS with preferential flow of microspheres to the hyperemic zone surrounding the ablated tissue (i.e., target volume). F Liver volume infused with 166Ho-MS TARE (i.e., treatment volume) [22].
On day 2, an angiography procedure was performed to selectively catheterize the hepatic arteries with vascular supply to the hyperemic tissue using a Progreat 2.4F or 2.7F microcatheter (Terumo corporation, Tokyo, Japan). Catheter position(s) were chosen as selectively as possible and were verified by contrast enhanced cone-beam CT (CBCT). Next, 150 MBq of technetium-99m labeled macroaggregated albumin ([99mTc]Tc-MAA) was injected. The treatment volume was defined as the volume exposed to radiation, based on CBCT [23]. This would include both the hyperemic zone (i.e., target volume) and a limited volume of normal liver parenchyma (i.e., non-target volume). A single photon emission computed tomography (SPECT-CT) scan was acquired directly after the angiography procedure on a Symbia T6 or Symbia Intevo (Siemens Healthineers, Erlangen, Germany) or Discovery 670 Pro (GE Healthcare, Boston, Massachusetts, USA).
On day 5–10 after RFA, TARE with infusion of 166Ho-MS QuiremSpheres (Quirem Medical B.V., Deventer, the Netherlands) was performed during a second hospitalization. Prior to 166Ho-MS injection, the catheter position was verified using fluoroscopy and CBCT to ensure that spheres would be injected at the identical location as the [99mTc]Tc-MAA. The total activity administered was calculated using the following equation [24]:
The treatment volume was segmented from the contrast enhanced CBCT and a tissue density of 1.00 g/mL was used to determine the mass of the treatment volume (Mi). One day after TARE (day 6–11), a post-treatment SPECT-CT was acquired for post-treatment dosimetry purposes. These SPECT images were acquired with a medium energy general purpose collimator. A total of 90 projections over a circular 360° orbit were acquired on a 128 × 128 matrix with an overall scanning time of 27 min (18 s per projection). Projections were recorded in the 81 keV (15% width) photopeak window. An additional energy window centered at 118 keV (12% width) was used to correct for bremsstrahlung and higher energy gamma emissions. Planar scintigraphy was used to calculate lung shunting. In addition to this SPECT-scan, MRI was performed before and after TARE to allow MRI-based quantification of 166Ho-MS. The MRI-images were acquired on a 1.5T scanner (Ingenia, Philips Healthcare, Best, The Netherlands) and included an MGRE sequence with 10 subsequent echoes (TE1, 1.06 ms; ∆TE, 1.38 ms; TR, 149 ms; flip angle, 33°; in-plane resolution, 2 × 2 mm2; slice thickness, 4 mm; FOV, 384 × 384 mm2).
Follow-up
All patients were followed for 12 months which included imaging using CECT or dynamic MRI of the liver and chest at 6 weeks and 3 months after treatment, and every 3 months thereafter. Clinical assessment and biochemical liver function tests were performed at week 2 and simultaneous with all moments of imaging.
Endpoints
The primary endpoint of this study was to find the treatment volume absorbed dose that resulted in an absorbed dose of ≥ 120 Gy to the target volume in 9/10 patients within a cohort, based on post-treatment SPECT-CT. The target volume was defined as the hyperemic zone encompassing the ablated tissue and generally anticipated to be a 1-cm rim around the ablated tissue. Manual segmentation of the treatment and target volumes in the post-treatment SPECT scan was performed using Xeleris workstation version 4.0 (GE Healthcare, Boston, Massachusetts, USA). The non-target volume dose was defined as the treatment volume subtracted by the target volume. Post-treatment MRI dosimetry was performed using Q-Suite 2.0 software (Quirem Medical B.V. Deventer, The Netherlands).
In the first cohort, a dose of 60 Gy was administered to the treatment volume. If a second patient within a cohort failed to reach an absorbed dose of ≥ 120 Gy to the target volume, the dose was escalated to 90 Gy to the treatment volume in subsequent patients (cohort 2) and could ultimately be escalated to 120 Gy (cohort 3). The design of this study was based on the assumption that microspheres would preferentially flow to the hyperemic zone around the ablation zone (i.e., target volume) rather than to the normal parenchyma (i.e., non-target volume) within the treatment volume. If the ratio of microsphere accumulation in the target volume versus normal non-target volume would be high, a low amount of radioactivity to the treatment volume (cohort 1) would be sufficient to reach an absorbed dose of ≥ 120 Gy to the target volume. If there would be an even distribution of microspheres between the target volume and non-target volume a treatment volume absorbed dose of 120 Gy (cohort 3) would be needed to meet the study endpoint. Per cohort at least 2 patients were treated and no further dose escalation was performed when the final endpoint was met of an absorbed dose of ≥ 120 Gy to the target volume in 9/10 patients. The sample size of this study was thus determined to be a minimum of 10 and a maximum of 30 patients.
Secondary endpoints included toxicity, local tumor recurrence rates, progression-free survival (PFS), and overall survival (OS) at 6 months and at 1 year. Adverse events were categorized according to Common Terminology Criteria for Adverse Events (CTCAE) 4.0 [25]. Local recurrences were defined as appearance at follow-up of foci of untreated disease in tumors that were previously considered to be completely ablated, in concordance with the CIRSE Standards of practice guideline [26].
MRI-based quantification of 166Ho-MS was investigated as an exploratory endpoint.
Statistical analysis
Descriptive statistics and outcomes were calculated by medians and interquartile ranges (IQR) for continuous variables and frequencies and percentages per category for categorical variables. Local recurrence free survival, PFS, and OS rates at 6-month and 12-month follow-up were calculated. Patients that underwent liver transplantation were censored in the survival statistics. Statistical analyses were performed using RStudio 1.4.1106.
Results
Patients
Informed consent was obtained from 20 patients between April 2018 and March 2021. Twelve of these patients completed the treatment regimen, as can be seen in Fig. 2. Reasons for exclusion were withdrawal from the study (n = 3), progression beyond BCLC early stage HCC in the time between inclusion and treatment (n = 1), CTCAE grade 3 complication after RFA (n = 1), RFA off target (n = 1), high lung shunt fraction (n = 1), and incomplete administration of 166Ho-MS (n = 1). Baseline characteristics of all 12 treated patients are shown in Table 2. The population consisted of more males (n = 10) than females (n = 2) and most patients had underlying Child-Pugh A liver cirrhosis (n = 10).
Treatment
A patient case example is given in Fig. 3. Treatment characteristics can be found in Table 3. All ablations were performed with a multiprobe approach. Three out of 16 lesions in two out of 12 patients were treated with RFA only as the tumor diameter was < 2 cm. In those patients, only the larger lesion(s) (> 2 cm) were treated with adjuvant TARE after RFA. Most 166Ho-MS infusions were performed (sub-)segmental or bi-segmental, and one infusion was performed lobar. The median treatment volume was 360 mL (IQR, [270–394]), and the median administered activity of 166Ho was 1.79 GBq (IQR, [1.45–2.23]).
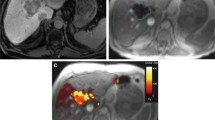
HORA EST HCC treatment sequence: A Arterial scan phase of diagnostic MRI showing a hypervascular HCC lesion of 31 mm in the liver. B Portal venous scan phase of MRI showing central wash-out in the HCC lesion. C Intraprocedural CT after placement of six cooled-tip RFA needles with 3-cm exposed tip. D Intraprocedural contrast enhanced CT scan in arterial phase showing hyperemia around the ablation zone on post-ablation CECT. E Super-selective catheterization of hepatic arteries with vascular supply to the target volume. F CBCT of the treatment volume with an identical catheter position as in E. G SPECT-CT of [99mTc]Tc-MAA dose distribution used for dose planning. H SPECT-CT of 166Ho-MS distribution. I MRI-based dosimetry of 166Ho-MS distribution.
Primary endpoint
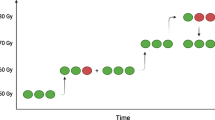
The first two patients were treated with a dose of 60 Gy on the treatment volume. Figure 4 shows the dose distribution per patient. Although a preferential dose accumulation in the target volume was found in the first two patients, the absorbed target volume doses were 89 Gy and 93 Gy, respectively. As the endpoint of ≥ 120 Gy to the target volume was not met, the dose was escalated to 90 Gy to the treatment volume. In 9/10 patients in the 90 Gy cohort, a mean target volume dose of ≥ 120 Gy was met. In this cohort the median absorbed target volume dose was 138 Gy (IQR, [127–145]), and the median absorbed non-target volume dose was 67 Gy (IQR, [54–75]), as can be seen in Fig. 4. As the primary endpoint was met, the inclusion was closed and the recommended treatment volume absorbed dose was set at 90 Gy.
Dose distribution per patient within treatment volume, based on SPECT imaging. The bars in black represent the mean absorbed dose on the target volume directly surrounding the ablation volume per patient. The cutoff point of an absorbed target volume dose of \(\ge\) 120 Gy is indicated by the horizontal dashed line. The bars in white show the absorbed dose to the non-target volume within the treatment volume. The first two patients were treated with 60 Gy to the treatment volume, whereas the other patients were treated with 90 Gy to the treatment volume. The median ratio of target volume dose vs non-target volume dose was 1.97 (IQR, [1.75–2.17]).
Toxicity
One patient was readmitted to the hospital on the third day after radioembolization because of fever. Ultrasound and CECT demonstrated abscess formation within the ablated tissue that was treated with percutaneous drainage (CTCAE 4.0 grade 3 infection). Other reported adverse events were grade 1–2 nausea (n = 3) and grade 1 fatigue (n = 4).
Efficacy
Two patients underwent liver transplantation at 7.5 and 8.0 months after treatment. They were both local recurrence free before liver transplantation. All other ten patients were also free of local recurrences within 12 months after treatment. Three patients developed new HCC lesions elsewhere in the liver, at 4.6, 5.5, and 5.6 months. Therefore, PFS was 75% at 6 months and 75% at 1 year. Two patients died, one as a result of decompensated liver cirrhosis and one following bacterial sepsis after liver transplantation. This resulted in an OS of 92% at 6 months and 83% at 1 year. Figure 5 shows an example of histological confirmation of 166Ho-MS accumulation surrounding the fibrotic and central necrotic tissue.
Histology of explanted liver treated with radiofrequency ablation and adjuvant 166Ho TARE. Digitalized histology using Ultra Fast Scanner (Philips Healthcare, Best, The Netherlands) with a magnitude of 40×. A Zoom 10×. Transition from liver tissue with ductal proliferation to fibrosis with marked depositions of 166Ho-MS. B Zoom 5×. Overview of transition from ductal proliferation to necrotic tissue with marked 166Ho-MS.
Discussion
In this multi-center, single arm study we prospectively evaluated the feasibility of adjuvant TARE after RFA in BCLC early stage HCC 2–5 cm. The results show that an absorbed dose of > 120 Gy of 166Ho-MS on the target volume around the ablation zone could be reached at an administered dose of 90 Gy to the treatment volume. The median target volume dose was about twice as high as the median dose to the non-target parenchyma, confirming our hypothesis that hyperemia induced by RFA can be utilized to deposit 166Ho-MS in a peripheral zone surrounding the ablation volume. The safety profile of the combined treatment was in concordance with the safety of RFA or TARE mono-therapy, or combined RFA and TACE. Only one CTCAE grade 4 complication occurred in 12 patients (8.3%) and no grade 5 complications were observed [27,28,29]. Within 1 year after treatment, no local recurrences developed, three patients developed recurrent HCC elsewhere in the liver and two patients died. Treatment efficacy and safety profile should be further validated in a larger cohort.
Many patients with larger HCC lesions are not eligible to surgical resection due to comorbidities, cirrhosis with portal hypertension, or insufficient future liver remnant volume. TA is an alternative treatment, but a large diameter is an important risk factor of local recurrence [6, 8]. In the continuous search towards better treatment outcomes and extended bridging to liver transplantation, several treatment combinations of TA with other locoregional or systemic therapies have been investigated. The STORM trial investigated adjuvant sorafenib after surgery or TA, but failed to prove benefit in terms of time to progression free and overall survival [30]. Another widely studied combined treatment regimen is TA with (neo)adjuvant TACE. Several trials in Asian populations have indicated superiority of combined TA and TACE over TA alone [31, 32], but the combination therapy has not been adopted in the EASL, AASL, or BCLC guidelines [1, 2, 7]. The different studies have methodological limitations and there is a considerable variation between the trials in technique and treatment sequence [33,34,35]. Furthermore, superiority of the combination therapy over surgical resection has not been proven [11, 33]. To our knowledge this is the first study to combine TA with TARE.
Technical advancements have led to the adoption of TA as the preferred treatment of HCC < 2 cm a decade ago [2, 36]. Similarly, recent advancements in patient selection and optimized patient-tailored dosing have resulted in a place for TARE in the recent BCLC update [2]. The LEGACY and RASER studies reported promising results of radiation segmentectomy in patients with (very) early stage HCC patients with a mean lesion diameter of 2.7 cm and median lesion diameter of 2.1 cm, respectively [37, 38]. These results indicate high local control rates to be achievable using radiation segmentectomy, although results were not superior to those that may be achieved with TA. Further prospective validation is needed in larger trials and in patients with larger lesions. Ultimately, the role of TARE in HCC is to be further clarified for different indications.
In this trial, only RFA was used as ablation modality. In this way, the treatment regimen was kept as homogeneous as possible. Moreover, preclinical work combining TA with radiation-based therapies was only performed with RFA [12,13,14,15]. However, over the last years, the use of microwave ablation (MWA) has increased. MWA may have some technological advancements over RFA, but similar outcomes have been found [39]. As hyperemia around the ablation zone is seen after MWA similarly to RFA, it is expected that a similar 166Ho-MS dose distribution can be achieved when TARE is performed following MWA [40,41,42].
166Ho-MS were used for radioembolization in this study rather than 90Y TARE. 166Ho has advantages in terms of imaging as it emits direct gamma radiation at 81 keV. Moreover, the paramagnetic property of 166Ho allows for MRI-based post TARE dosimetry [24, 43]. The study endpoint was determined using SPECT-based dosimetry, and MRI-based quantification of 166Ho-MS was used as an exploratory endpoint. Unfortunately, reliable quantitative MRI-based dosimetry was unfeasible in many patients as a result of breathing and movement artifacts. MRI scans were obtained shortly after the RFA and TARE procedures, and many patients experienced discomfort and as a result had difficulty lying still and maintaining breath holds. [99mTc]Tc-MAA was used for the scout procedure. Despite the potential benefits of 166Ho scout dose in terms of intrahepatic treatment dose distribution mimicking, 166Ho scout dose was not yet available by the time of study design [44]. In the current study, however, standard volume-based dosimetry was used based on CBCT, so this would not have affected dose planning
The current study has several limitations. First, the sample size is small and therefore no definite conclusions can be drawn on the efficacy. Nevertheless, the absence of local recurrences in all study patients within 1 year after treatment suggests that the efficacy of the combination therapy is high. Second, despite of meeting the primary end point at an administered dose of 90 Gy, a substantial variety in ratio of target volume dose versus non-target volume dose between individual patients was observed. This ratio depends on various factors, such as degree of hyperemia, catheter position, occurrence of vascular stasis during injection, and the ratio target volume versus treatment volume. In the 90 Gy cohort, an absorbed target volume dose of ≥ 120 Gy was not reached in one patient. As a result of a very selective catheter position in this patient, the target volume constituted > 50% of the treatment volume. In patients where the ratio between target volume and treatment volume ratio is very high, an administered dose higher than 90 Gy to the treatment volume may be required. Clearly, in the theoretical case that the target volume constitutes 100% of the treatment volume, TARE with a dose of 90 Gy would not be sufficient. For future studies, a volume-dependent administration dose planning could help to individualize treatment planning. Another limitation of this study is the complexity of the treatment regimen. For patients this meant undergoing a second treatment, including four additional imaging examinations (2× MRI and 2× SPECT/CT) and an additional hospitalization. This was considered burdensome by some patients, and therefore a reason not to participate in this trial.
Since the initial plans of this study originate from 2017, less was known on (166Ho) TARE dosimetry, and the 120 Gy cutoff was mainly chosen based on initial 90Y research [21]. Treatment volume absorbed doses of the several cohorts were based on a phase I 166Ho-MS dose escalation study (HEPAR trial), in which a whole liver dose of 60 Gy was considered safe [45]. Recently, the first efficacy evidence for 166Ho-MS in HCC was demonstrated in the HEPAR Primary study [28]. At a treatment volume absorbed dose of 50 Gy in an average of 54% of the total liver volume, partial or complete responses were seen in patients receiving an average absorbed dose of 210 Gy on their lesions versus 116 Gy in patients with progressive disease [28]. Since hyperemic tissue surrounding the ablation zone is targeted in our study rather than (large) lesions, these tumor dose values cannot be directly compared to the 138 Gy absorbed target volume dose found in our study. Nevertheless, taking into account the recent advancements of safe radiation segmentectomy procedures, and the fact that a tumor absorbed dose of 210 Gy did show a higher level of tissue necrotization when compared to an absorbed dose of 116 Gy in the HEPAR primary study, investigating higher dosing of 166Ho-MS as adjuvant treatment after thermal ablation seems to be justified. Especially when a small treatment volume is treated that mainly consists of the target volume, a higher dose than in the current trail should be chosen. In light of recent segmentectomy studies [37, 38, 46], recommendations with 90Y [47], and the HEPAR primary trial [28], the treatment volume absorbed dose may be as high as about 200 Gy. For patients with a larger treatment volume (for example due to multiple ablations or a more centrally located tumor), a treatment volume absorbed dose of 90 Gy remains recommended to limit the absorbed radiation dose to the liver parenchyma. Our study provides insight in the biodistribution of 166Ho-MS after TA with an average target volume vs non-target volume ratio of 2:1. This may help to determine the optimal dose in each individual patient, while taking into account the risk of radiation induced liver disease in patients with a larger treatment volume.
The median tumor diameter in this study was 2.7 cm. Patients with a tumor diameter of ≥ 2 cm were eligible for inclusion in this dose finding study. It may be questionable whether adjuvant TARE will be cost-effective in patients with a tumor < 3 cm. Future studies investigating effectivity of thermal ablation with adjuvant TARE are more likely to be positive when larger tumors are recruited.
Moreover, in a future study, the feasibility of combined TA and TARE in a single procedure could be explored. Owing to the low dose of 166Ho-MS used in this treatment regimen, the chance of introducing a substantial radiation dose to the lung parenchyma is extremely low. Moreover, as a result of super-selective catheterization and the use of CBCT prior to infusion of 166Ho-MS, the chance of other extrahepatic deposition is small as well. Especially since combined Angio-CT systems are increasingly being used, the combined treatment could be performed in a single session with high precision [48]. The current proposed treatment protocol is promising for the locoregional treatment of HCC lesions 2–5 cm that are at higher risk of local recurrences. Further research into subtypes of HCC or identification of satellite nodules may contribute to identifying patients who potentially benefit most of the combined treatment regimen.
Conclusion
Selective radioembolization with 166Ho-MS can be used safely as an adjuvant treatment in early stage HCC 2–5 cm. Hyperemia induced by TA can be utilized to deliver a high radiation dose to the target volume while limiting the dose to the normal liver parenchyma. A treatment volume absorbed dose of 90 Gy is safe and sufficient to deliver a tumoricidal absorbed radiation dose of at least 120 Gy to the target volume.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Galle PR, Forner A, Llovet JM, Mazzaferro V, Piscaglia F, Raoul J-L, et al. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236.
Reig M, Forner A, Rimola J, Ferrer-Fábrega J, Burrel M, Garcia-Criado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76(3):681–93. https://doi.org/10.1016/j.jhep.2021.11.018.
Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–14.
Weis S, Franke A, Mössner J, Jakobsen JC, Schoppmeyer K. Radiofrequency (thermal) ablation versus no intervention or other interventions for hepatocellular carcinoma. Cochrane Database Syst Rev. 2013;(12):Cd003046. https://doi.org/10.1002/14651858.CD003046.pub3.
Xu X-L, Liu X-D, Liang M, Luo B-M. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma: systematic review of randomized controlled trials with meta-analysis and trial sequential analysis. Radiology. 2017;287:461–72.
Shin SW, Ahn KS, Kim SW, Kim TS, Kim YH, Kang KJ. Liver Resection Versus Local Ablation Therapies for Hepatocellular Carcinoma Within the Milan Criteria: A Systematic Review and Meta-analysis. Ann Surg. 2021;73(4):656–66. https://doi.org/10.1097/SLA.0000000000004350.
Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology (Baltimore, Md). 2018;67:358–80.
Burgmans MC, Too CW, Fiocco M, Kerbert AJC, Lo RHG, Schaapman JJ, et al. Differences in patient characteristics and midterm outcome between Asian and European patients treated with radiofrequency ablation for hepatocellular carcinoma. CardioVasc Intervent Radiol. 2016;39:1708–15.
Habibollahi P, Sheth RA, Cressman ENK. Histological Correlation for Radiofrequency and Microwave Ablation in the Local Control of Hepatocellular Carcinoma (HCC) before Liver Transplantation: A Comprehensive Review. Cancers (Basel). 2020;13(1):104. https://doi.org/10.3390/cancers13010104.
Cao S, Zou Y, Lyu T, Fan Z, Guan H, Song L, et al. Long-term outcomes of combined transarterial chemoembolization and radiofrequency ablation versus RFA monotherapy for single hepatocellular carcinoma ≤3 cm: emphasis on local tumor progression. Int J Hyperthermia. 2022;39:1–7.
Lin C-W, Chen Y-S, Lo G-H, Hsu Y-C, Hsu C-C, Wu T-C, et al. Comparison of overall survival on surgical resection versus transarterial chemoembolization with or without radiofrequency ablation in intermediate stage hepatocellular carcinoma: a propensity score matching analysis. BMC Gastroenterol. 2020;20:99.
Solazzo S, Mertyna P, Peddi H, Ahmed M, Horkan C, Nahum Goldberg S. RF ablation with adjuvant therapy: comparison of external beam radiation and liposomal doxorubicin on ablation efficacy in an animal tumor model. Int J Hyperthermia. 2008;24:560–7.
Horkan C, Dalal K, Coderre JA, Kiger JL, Dupuy DE, Signoretti S, et al. Reduced tumor growth with combined radiofrequency ablation and radiation therapy in a rat breast tumor model. Radiology. 2005;235:81–8.
Lin ZY, Chen J, Deng XF. Treatment of hepatocellular carcinoma adjacent to large blood vessels using 1.5T MRI-guided percutaneous radiofrequency ablation combined with iodine-125 radioactive seed implantation. Eur J Radiol 2012;81:3079-3083.
Chen K, Chen G, Wang H, Li H, Xiao J, Duan X, et al. Increased survival in hepatocellular carcinoma with iodine-125 implantation plus radiofrequency ablation: a prospective randomized controlled trial. J Hepatol. 2014;61:1304–11.
Dawson LA, Guha C. Hepatocellular Carcinoma: Radiation Therapy. The Cancer Journal. 2008;14(2):111–6. https://doi.org/10.1097/PPO.0b013e31816a0e80.
Cheng JC, Wu JK, Lee PC, Liu HS, Jian JJ, Lin YM, et al. Biologic susceptibility of hepatocellular carcinoma patients treated with radiotherapy to radiation-induced liver disease. Int J Radiat Oncol Biol Phys. 2004;60:1502–9.
Riaz A, Gates VL, Atassi B, Lewandowski RJ, Mulcahy MF, Ryu RK, et al. Radiation segmentectomy: a novel approach to increase safety and efficacy of radioembolization. Int J Radiat Oncol Biol Phys. 2011;79:163–71.
Roosen J, Klaassen NJM, Westlund Gotby LEL, Overduin CG, Verheij M, Konijnenberg MW, et al. To 1000 Gy and back again: a systematic review on dose-response evaluation in selective internal radiation therapy for primary and secondary liver cancer. Eur J Nucl Med Mol Imaging. 2021;48(12):3776–90. https://doi.org/10.1007/s00259-021-05340-0.
Park M-h, Rhim H, Kim Y-s, Choi D, Lim HK, Lee WJ. Spectrum of CT findings after radiofrequency ablation of hepatic tumors. RadioGraphics 2008;28:379-390.
Lau WY, Leung WT, Ho S, Leung NW, Chan M, Lin J, et al. Treatment of inoperable hepatocellular carcinoma with intrahepatic arterial yttrium-90 microspheres: a phase I and II study. Br J Cancer. 1994;70:994–9.
Hendriks P, Rietbergen DDD, van Erkel AR, Coenraad MJ, Arntz MJ, Bennink RJ, et al. Study protocol: adjuvant holmium-166 radioembolization after radiofrequency ablation in early-stage hepatocellular carcinoma patients—a dose-finding study (HORA EST HCC Trial). CardioVasc Intervent Radiol. 2022;45:1057–63.
Sabet A, Ahmadzadehfar H, Muckle M, Haslerud T, Wilhelm K, Biersack HJ, et al. Significance of oral administration of sodium perchlorate in planning liver-directed radioembolization. J Nucl Med Off Publ Soc Nucl Med. 2011;52:1063–7.
Smits ML, Elschot M, van den Bosch MA, van de Maat GH, van het Schip AD, Zonnenberg BA, et al. In vivo dosimetry based on SPECT and MR imaging of 166Ho-microspheres for treatment of liver malignancies. J Nucl Med Off Publ Soc Nucl Med. 2013;54:2093–100.
USA NIH National Cancer Institute. Common terminology criteria in adverse events, version 4.0 (CTCAE 4.0). [cited; Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm. Accessed 06 2017.
Crocetti L, de Baére T, Pereira PL, Tarantino FP. CIRSE standards of practice on thermal ablation of liver tumours. CardioVasc Intervent Radiol. 2020;43:951–62.
Kong WT, Zhang WW, Qiu YD, Zhou T, Qiu JL, Zhang W, et al. Major complications after radiofrequency ablation for liver tumors: analysis of 255 patients. World J Gastroenterol. 2009;15:2651–6.
Reinders MTM, van Erpecum KJ, Smits MLJ, Braat AJAT, Bruijne Jd, Bruijnen R, et al. Safety and efficacy of 166Ho radioembolization in hepatocellular carcinoma: the HEPAR primary study. J Nucl Med. 2022;63:1891.
Liu W, Xu H, Ying X, Zhang D, Lai L, Wang L, et al. Radiofrequency ablation (RFA) combined with transcatheter arterial chemoembolization (TACE) for patients with medium-to-large hepatocellular carcinoma: a retrospective analysis of long-term outcome. Med Sci Monit. 2020;26: e923263.
Bruix J, Takayama T, Mazzaferro V, Chau GY, Yang J, Kudo M, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16:1344–54.
Yin X, Zhang L, Wang Y-H, Zhang B-H, Gan Y-H, Ge N-L, et al. Transcatheter arterial chemoembolization combined with radiofrequency ablation delays tumor progression and prolongs overall survival in patients with intermediate (BCLC B) hepatocellular carcinoma. BMC Cancer. 2014;14:849.
Yamanaka T, Yamakado K, Takaki H, Nakatsuka A, Shiraki K, Hasegawa H, et al. Ablative zone size created by radiofrequency ablation with and without chemoembolization in small hepatocellular carcinomas. Jpn J Radiol. 2012;30:553–9.
Hendriks P, Sudiono DR, Schaapman JJ, Coenraad MJ, Tushuizen ME, Takkenberg RB, et al. Thermal ablation combined with transarterial chemoembolization for hepatocellular carcinoma: what is the right treatment sequence? Eur J Radiol. 2021;144: 110006.
Chen Q-W, Ying H-F, Gao S, Shen Y-H, Meng Z-Q, Chen H, et al. Radiofrequency ablation plus chemoembolization versus radiofrequency ablation alone for hepatocellular carcinoma: a systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2016;40:309–14.
Peng Z-W, Zhang Y-J, Chen M-S, Xu L, Liang H-H, Lin X-J, et al. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol. 2013;31:426–32.
Ng KKC, Chok KSH, Chan ACY, Cheung TT, Wong TCL, Fung JYY, et al. Randomized clinical trial of hepatic resection versus radiofrequency ablation for early-stage hepatocellular carcinoma. Br J Surg. 2017;104:1775–84.
Salem R, Johnson GE, Kim E, Riaz A, Bishay V, Boucher E, et al. Yttrium-90 radioembolization for the treatment of solitary, unresectable HCC: the LEGACY study. Hepatology (Baltimore, Md). 2021;74:2342–52.
Kim E, Sher A, Abboud G, Schwartz M, Facciuto M, Tabrizian P, et al. Radiation segmentectomy for curative intent of unresectable very early to early stage hepatocellular carcinoma (RASER): a single-centre, single-arm study. Lancet Gastroenterol Hepatol. 2022;7:843–50.
Poulou LS, Botsa E, Thanou I, Ziakas PD, Thanos L. Percutaneous microwave ablation vs radiofrequency ablation in the treatment of hepatocellular carcinoma. World J Hepatol. 2015;7:1054–63.
Carrafiello G, Laganà D, Mangini M, Fontana F, Dionigi G, Boni L, et al. Microwave tumors ablation: Principles, clinical applications and review of preliminary experiences. Int J Surg. 2008;6:S65–9.
Giorgio A, Gatti P, Montesarchio L, Merola MG, Amendola F, Calvanese A, et al. Microwave ablation in intermediate hepatocellular carcinoma in cirrhosis: an Italian multicenter prospective study. J Clin Transl Hepatol. 2018;6:251–7.
Vogl TJ, Nour-Eldin NA, Hammerstingl RM, Panahi B, Naguib NNN. Microwave ablation (MWA): basics, technique and results in primary and metastatic liver neoplasms - review article. Rofo. 2017;189:1055–66.
Roosen J, van Wijk MWM, Westlund Gotby LEL, Arntz MJ, Janssen MJR, Lobeek D, et al. Improving MRI-based dosimetry for holmium-166 transarterial radioembolization using a nonrigid image registration for voxelwise ΔR2* calculation. Med Phys. 2023;50:935–46.
Smits MLJ, Dassen MG, Prince JF, Braat A, Beijst C, Bruijnen RCG, et al. The superior predictive value of (166)Ho-scout compared with (99m)Tc-macroaggregated albumin prior to (166)Ho-microspheres radioembolization in patients with liver metastases. Eur J Nucl Med Mol Imaging. 2020;47:798–806.
Smits MLJ, Nijsen JFW, van den Bosch MAAJ, Lam MGEH, Vente MAD, Mali WPTM, et al. Holmium-166 radioembolisation in patients with unresectable, chemorefractory liver metastases (HEPAR trial): a phase 1, dose-escalation study. Lancet Oncol. 2012;13:1025–34.
Lewandowski RJ, Gabr A, Abouchaleh N, Ali R, Al Asadi A, Mora RA, et al. Radiation segmentectomy: potential curative therapy for early hepatocellular carcinoma. Radiology. 2018;287:1050–8.
Salem R, Padia SA, Lam M, Chiesa C, Haste P, Sangro B, et al. Clinical, dosimetric, and reporting considerations for Y-90 glass microspheres in hepatocellular carcinoma: updated 2022 recommendations from an international multidisciplinary working group. Eur J Nucl Med Mol Imaging. 2023;50:328–43.
Taiji R, Lin EY, Lin Y-M, Yevich S, Avritscher R, Sheth RA, et al. Combined angio-CT systems: a roadmap tool for precision therapy in interventional oncology. Radiol Imaging Cancer 2021;3:e210039.
Acknowledgements
The authors acknowledge the Dutch Liver Patients Association (Nederlandse Leverpatiënten Vereniging, NLV) and Dutch Oncology Research Platform DORP for contribution to the design of the trial. Moreover, the authors would like to thank Gerda Labadie and Bianca van Duin-de Vreugd for their trial management support and Gerrit Kracht for the design of Fig. 1.
Funding
This study was funded by a public-private partnership (PPP) allowance of the Dutch top consortium of knowledge and innovation (TKI): Life Science and Health (LSH). Project reference number: 40-41200-98-9286
Partners within this consortium were as follows:
1. Health~Holland (research grant)
2. Maag Lever Darm Stichting (MLDS): Dutch foundation for stomach, liver, and bowel disease (research grant)
3. Quirem Medical B.V. (in kind contribution of QuiremSpheres and Q-Suite software)
4. Medtronic, Inc (supported with a financial contribution from the Medtronic External Research Program)
5. Academic Medical Center, Amsterdam, The Netherlands (in kind)
6. Leiden University Medical Center, Leiden, The Netherlands (in kind)
Author information
Authors and Affiliations
Consortia
Contributions
Conceptualization: Mark C. Burgmans, Pim Hendriks, Arian R. van Erkel, Minneke J. Coenraad, Lioe-Fee de Geus-Oei. Methodology: Mark C. Burgmans, Pim Hendriks, Arian R. van Erkel, Minneke J. Coenraad, Lioe-Fee de Geus-Oei. Validation: Pim Hendriks, Daphne DD Rietbergen, Petra Dibbets-Schneider, Lioe-Fee de Geus-Oei, Mark C. Burgmans. Formal analysis: Pim Hendriks, Mark C. Burgmans. Investigation: Pim Hendriks, Daphne DD Rietbergen, Petra Dibbets-Schneider, Lioe-Fee de Geus-Oei, Mark C. Burgmans. Resources: Pim Hendriks, Daphne DD Rietbergen, Arian R. van Erkel, Minneke J. Coenraad, Mark J. Arntz, Roel J. Bennink, Andries E. Braat, Stijn Crobach, Otto M. van Delden, Petra Dibbets-Schneider, Tom van der Hulle, Heinz-Josef Klümpen, Rutger W. van der Meer, J. Frank W. Nijsen, Catharina SP van Rijswijk, Joey Roosen, Bastian N. Ruijter, Frits Smit, Mette K. Stam, R. Bart Takkenberg, Maarten E. Tushuizen, Floris HP van Velden, Lioe-Fee de Geus-Oei, Mark C. Burgmans. Data curation: Pim Hendriks, Mark C. Burgmans. Writing—original draft: Pim Hendriks. Writing—review and editing: Daphne DD Rietbergen, Arian R. van Erkel, Minneke J. Coenraad, Mark J. Arntz, Roel J. Bennink, Andries E. Braat, Stijn Crobach, Otto M. van Delden, Petra Dibbets-Schneider, Tom van der Hulle, Heinz-Josef Klümpen, Rutger W. van der Meer, J. Frank W. Nijsen, Catharina SP van Rijswijk, Joey Roosen, Bastian N. Ruijter, Frits Smit, Mette K. Stam, R. Bart Takkenberg, Maarten E. Tushuizen, Floris HP van Velden, Lioe-Fee de Geus-Oei, Mark C. Burgmans. Visualization: Pim Hendriks, Stijn Crobach, Mark Burgmans, Lioe-Fee de Geus-Oei. Supervision: Mark C. Burgmans, Daphne DD Rietbergen, Lioe-Fee de Geus-Oei. Project administration: Pim Hendriks, Mark C. Burgmans. Funding acquisition: Mark C. Burgmans, Arian R. van Erkel, Minneke J. Coenraad, Lioe-Fee de Geus-Oei.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Committee of Medical Ethics of the Leiden University Medical Center (Date: 18-01-2018; Number P17.161).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
The authors affirm that human research participants provided informed consent for publication of the images in Figs. 3 and 5.
Competing interests
M.C. Burgmans has received an educational grant from Boston Scientific and Medtronic and consultancy fees from SIRTeX, Medtronic, Delcath Systems, and Philips Health Care. None are related to the current project. J.F.W. Nijsen is co-founder of Quirem Medical, which has been acquired by Terumo Europe NV in July 2020. Nijsen has a scientific advisory role and is entitled to certain milestone payments from Terumo, which are related to Quirem’s financial, operational, and regulatory performance in the future. Furthermore, Nijsen is inventor on the patents related to radioactive microspheres that are assigned to University Medical Center Utrecht Holding BV, Quirem Medical, or BASF Corp. The activities of J.F.W. Nijsen within Quirem Medical are approved and supported by the Board of Directors of the Radboudumc. All other authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hendriks, P., Rietbergen, D.D.D., van Erkel, A.R. et al. Adjuvant holmium-166 radioembolization after radiofrequency ablation in early-stage hepatocellular carcinoma patients: a dose-finding study (HORA EST HCC trial). Eur J Nucl Med Mol Imaging (2024). https://doi.org/10.1007/s00259-024-06630-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00259-024-06630-z