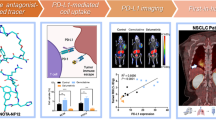
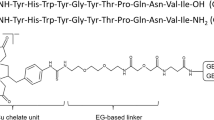
Abstract
Purpose
Neuropilin-1 (NRP-1) is a multifunctional protein involved in a variety of biological processes such as angiogenesis, tumorigenesis and immunomodulation. It was usually overexpressed in many cancer cell lines and correlated with poor prognosis of breast cancer. Positron emission tomography (PET) is an advanced imaging technique for detecting the function and metabolism of tumor-associated molecules in real time, dynamically, quantitatively and noninvasively. To improve the level of early diagnosis and evaluate the prognosis of breast cancer, an NRP-1 targeting peptide-based tracer [68 Ga]Ga-NOTA-PEG4-CK2 was designed to sensitively and specifically detect the NRP-1 expression in vivo via PET imaging.
Methods
In silico modeling and microscale thermophoresis (MST) assay were carried out to design the NRP-1 targeting peptide NOTA-PEG4-CK2, and it was further radiolabeled with 68 Ga to prepare the tracer [68 Ga]Ga-NOTA-PEG4-CK2. The radiochemical yield (RCY), radiochemical purity (RCP), molar activity (Am), lipid-water partition coefficient (Log P) and stability of [68 Ga]Ga-NOTA-PEG4-CK2 were assessed. The targeting specificity of the tracer for NRP-1 was investigated by in vitro cellular uptake assay and in vivo PET imaging as well as blocking studies. The sensitivity of the tracer in monitoring the dynamic changes of NRP-1 expression induced by chemical drug was also investigated in vitro and in vivo. Ex vivo biodistribution, autoradiography, western blot, and immunofluorescence staining were also performed to study the specificity of [68 Ga]Ga-NOTA-PEG4-CK2 for NRP-1.
Results
[68 Ga]Ga-NOTA-PEG4-CK2 was designed and synthesized with high RCY (> 98%), high stability (RCP > 95%) and high affinity to NRP-1 (KD = 25.39 ± 1.65 nM). In vitro cellular uptake assay showed that the tracer [68 Ga]Ga-NOTA-PEG4-CK2 can specifically bind to NRP-1 positive cancer cells MDA-MB-231 (1.04 ± 0.04% at 2 h) rather than NRP-1 negative cancer cells NCI-H1299 (0.43 ± 0.05%). In vivo PET imaging showed the maximum tumor uptake of [68 Ga]Ga-NOTA-PEG4-CK2 in MDA-MB-231 xenografts (4.16 ± 0.67%ID/mL) was significantly higher than that in NCI-H1299 xenografts (1.03 ± 0.19%ID/mL) at 10 min post injection, and the former exhibited higher tumor-to-muscle uptake ratio (5.22 ± 0.18) than the latter (1.07 ± 0.27) at 60 min post injection. MDA-MB-231 xenografts pretreated with nonradioactive precursor NOTA-PEG4-CK2 showed little tumor uptake of [68 Ga]Ga-NOTA-PEG4-CK2 (1.67 ± 0.38%ID/mL at 10 min post injection). Both cellular uptake assay and PET imaging revealed that NRP-1 expression in breast cancer MDA-MB-231 could be effectively suppressed by SB-203580 treatment and can be sensitively detected by [68 Ga]Ga-NOTA-PEG4-CK2. Ex vivo analysis also proved the high specificity and sensitivity of [68 Ga]Ga-NOTA-PEG4-CK2 for NRP-1 expression in MDA-MB-231 xenografts.
Conclusion
A promising NRP-1 targeting PET tracer [68 Ga]Ga-NOTA-PEG4-CK2 was successfully prepared. It showed remarkable specificity and sensitivity in monitoring the dynamic changes of NRP-1 expression. Hence, it could provide valuable information for early diagnosis of NRP-1 relevant cancers and evaluating the prognosis of cancer patients.
Graphical Abstract
A novel promising NRP-1 targeting PET tracer [68 Ga]Ga-NOTA-PEG4-CK2 was developed based on a series of in vitro and in vivo investigations. The tracer showed remarkable specificity and sensitivity in detecting the expression of NRP-1. It could be applied for noninvasively and dynamically monitoring the NRP-1 expression in tumors and predicting the prognosis of breast cancer.








Similar content being viewed by others
Data availability
Data generated in the present study are available from the corresponding authors upon reasonable request.
References
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. https://doi.org/10.3322/caac.21763.
Riggio AI, Varley KE, Welm AL. The lingering mysteries of metastatic recurrence in breast cancer. Br J Cancer. 2021;124(1):13–26. https://doi.org/10.1038/s41416-020-01161-4.
Madu CO, Wang S, Madu CO, Lu Y. Angiogenesis in breast cancer progression, diagnosis, and treatment. J Cancer. 2020;11(15):4474–94. https://doi.org/10.7150/jca.44313.
Liu ZL, Chen HH, Zheng LL, Sun LP, Shi L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct Target Ther. 2023;8(1):198. https://doi.org/10.1038/s41392-023-01460-1.
Dumond A, Pages G. Neuropilins, as relevant oncology target: their role in the tumoral microenvironment. Front Cell Dev Biol. 2020;8:662. https://doi.org/10.3389/fcell.2020.00662.
Zhao L, Chen H, Lu L, Wang L, Zhang X, Guo X. New insights into the role of co-receptor neuropilins in tumour angiogenesis and lymphangiogenesis and targeted therapy strategies. J Drug Target. 2021;29(2):155–67. https://doi.org/10.1080/1061186X.2020.1815210.
Naik A, Al-Zeheimi N, Bakheit CS, Al Riyami M, Al Jarrah A, Al Moundhri MS, et al. Neuropilin-1 associated molecules in the blood distinguish poor prognosis breast cancer: a cross-sectional study. Sci Rep. 2017;7(1):3301. https://doi.org/10.1038/s41598-017-03280-0.
Wang H, Zhang YN, Xu DQ, Huang JG, Lv D, Shi XY, et al. Neuropilin1, a novel independent prognostic factor and therapeutic target in triple-negative breast cancer. Neoplasma. 2020;67(6):1335–42. https://doi.org/10.4149/neo_2020_191127N1223.
Rachner TD, Kasimir-Bauer S, Goebel A, Erdmann K, Hoffmann O, Rauner M, et al. Soluble Neuropilin-1 is an independent marker of poor prognosis in early breast cancer. J Cancer Res Clin Oncol. 2021;147(8):2233–8. https://doi.org/10.1007/s00432-021-03635-1.
Al-Zeheimi N, Gao Y, Greer PA, Adham SA. Neuropilin-1 knockout and rescue confirms its role to promote metastasis in MDA-MB-231 breast cancer cells. Int J Mol Sci. 2023;24(9):7792. https://doi.org/10.3390/ijms24097792.
Liu SD, Zhong LP, He J, Zhao YX. Targeting neuropilin-1 interactions is a promising anti-tumor strategy. Chin Med J. 2021;134(5):508–17. https://doi.org/10.1097/CM9.0000000000001200.
Feng GK, Liu RB, Zhang MQ, Ye XX, Zhong Q, Xia YF, et al. SPECT and near-infrared fluorescence imaging of breast cancer with a neuropilin-1-targeting peptide. J Control Release. 2014;192:236–42. https://doi.org/10.1016/j.jconrel.2014.07.039.
Su H, Zhao L, Yu B, Zeng H, Yang J, Zhu M, et al. Preparation and bioevaluation of [99mTc]Tc-labeled A7R and DA7R for SPECT imaging of triple-negative breast cancer. New J Chem. 2022;46(44):21401–8. https://doi.org/10.1039/d2nj04136g.
Bumbaca D, Xiang H, Boswell CA, Port RE, Stainton SL, Mundo EE, et al. Maximizing tumour exposure to anti-neuropilin-1 antibody requires saturation of non-tumour tissue antigenic sinks in mice. Brit J Pharmacol. 2012;166(1):368–77. https://doi.org/10.1111/j.1476-5381.2011.01777.x.
Ma C, Dou X, Yan J, Wang S, Yang R, Su F, et al. Optimal saturated neuropilin-1 expression in normal tissue maximizes tumor exposure to anti-neuropilin-1 monoclonal antibody. Anticancer Agents Med Chem. 2019;19(18):2269–75. https://doi.org/10.2174/1871520619666191105150235.
Adhikari A, Tiwari AK, Shukla A, Mishra AK, Datta A. Synthesis and preclinical evaluation of radioligand, 99mTc-DO3A-Et-RPAR for imaging NRP-1 specific tumor. ChemistrySelect. 2019;4(44):12950–4. https://doi.org/10.1002/slct.201902556.
Dong P, Cai H, Chen L, Li Y, Yuan C, Wu X, et al. Biodistribution and evaluation of 131I-labeled neuropilin-binding peptide for targeted tumor imaging. Contrast Media Mol Imaging. 2016;11(6):467–74. https://doi.org/10.1002/cmmi.1708.
Wu H, Chen H, Pan D, Ma Y, Liang S, Wan Y, et al. Imaging integrin αvβ3 and NRP-1 positive gliomas with a novel fluorine-18 labeled RGD-ATWLPPR heterodimeric peptide probe. Mol Imaging Biol. 2014;16(6):781–92. https://doi.org/10.1007/s11307-014-0761-0.
Lu L, Chen H, Hao D, Zhang X, Wang F. The functions and applications of A7R in anti-angiogenic therapy, imaging and drug delivery systems. Asian J Pharm Sci. 2019;14(6):595–608. https://doi.org/10.1016/j.ajps.2019.04.004.
Moussaron A, Jouan-Hureaux V, Collet C, Pierson J, Thomas N, Choulier L, et al. Preliminary study of new gallium-68 radiolabeled peptide targeting NRP-1 to detect brain metastases by positron emission tomography. Molecules. 2021;26(23):7273. https://doi.org/10.3390/molecules26237273.
Yao L, Li Y, Chen H, Wen X, Pang Y, Chen Z, et al. Dual targeting of integrin αvβ3 and neuropilin-1 receptors improves micropositron emission tomography imaging of breast cancer. Mol Pharm. 2022;19(5):1458–67. https://doi.org/10.1021/acs.molpharmaceut.1c01015.
Maslowska K, Witkowska E, Tymecka D, Halik PK, Misicka A, Gniazdowska E. Synthesis, physicochemical and biological study of gallium-68- and lutetium-177-labeled VEGF-A165/NRP-1 complex inhibitors based on peptide A7R and branched peptidomimetic. Pharmaceutics. 2022;14(1):100. https://doi.org/10.3390/pharmaceutics14010100.
Hendrikx G, Voo S, Bauwens M, Post MJ, Mottaghy FM. SPECT and PET imaging of angiogenesis and arteriogenesis in pre-clinical models of myocardial ischemia and peripheral vascular disease. Eur J Nucl Med Mol Imaging. 2016;43(13):2433–47. https://doi.org/10.1007/s00259-016-3480-8.
Garcia-Figueiras R, Baleato-Gonzalez S, Padhani AR, Luna-Alcala A, Vallejo-Casas JA, Sala E, et al. How clinical imaging can assess cancer biology. Insights Imaging. 2019;10(1):28. https://doi.org/10.1186/s13244-019-0703-0.
Zhang K, Sun Y, Wu S, Zhou M, Zhang X, Zhou R, et al. Systematic imaging in medicine: a comprehensive review. Eur J Nucl Med Mol Imaging. 2021;48(6):1736–58. https://doi.org/10.1007/s00259-020-05107-z.
Teesalu T, Sugahara KN, Kotamraju VR, Ruoslahti E. C-end rule peptides mediate neuropilin-1-dependent cell, vascular, and tissue penetration. Proc Natl Acad Sci USA. 2009;106(38):16157–62. https://doi.org/10.1073/pnas.0908201106.
Roth L, Agemy L, Kotamraju VR, Braun G, Teesalu T, Sugahara KN, et al. Transtumoral targeting enabled by a novel neuropilin-binding peptide. Oncogene. 2012;31(33):3754–63. https://doi.org/10.1038/onc.2011.537.
Ciobanasu C, Dragomir I, Apetrei A. The penetrating properties of the tumor homing peptide LyP-1 in model lipid membranes. J Pept Sci. 2019;25(3): e3145. https://doi.org/10.1002/psc.3145.
Goudiaby I, Malliavin TE, Mocchetti E, Mathiot S, Acherar S, Frochot C, et al. New crystal form of human neuropilin-1 b1 fragment with six electrostatic mutations complexed with KDKPPR peptide ligand. Molecules. 2023;28(14):5603. https://doi.org/10.3390/molecules28145603.
Larue L, Kenzhebayeva B, Al-Thiabat MG, Jouan-Hureaux V, Mohd-Gazzali A, Wahab HA, et al. tLyp-1: a peptide suitable to target NRP-1 receptor. Bioorg Chem. 2023;130: 106200. https://doi.org/10.1016/j.bioorg.2022.106200.
Parikh AA, Fan F, Liu WB, Ahmad SA, Stoeltzing O, Reinmuth N, et al. Neuropilin-1 in human colon cancer: expression, regulation, and role in induction of angiogenesis. Am J Pathol. 2004;164(6):2139–51. https://doi.org/10.1016/S0002-9440(10)63772-8.
Yu DC, Waby JS, Chirakkal H, Staton CA, Corfe BM. Butyrate suppresses expression of neuropilin I in colorectal cell lines through inhibition of Sp1 transactivation. Mol Cancer. 2010;9:276–8. https://doi.org/10.1186/1476-4598-9-276.
Lee J, Kim E, Ryu SW, Choi C, Choi K. Combined inhibition of vascular endothelial growth factor receptor signaling with temozolomide enhances cytotoxicity against human glioblastoma cells via downregulation of neuropilin-1. J Neurooncol. 2016;128(1):29–34. https://doi.org/10.1007/s11060-016-2091-3.
Al-Zeheimi N, Naik A, Bakheit CS, Al Riyami M, Al Ajarrah A, Al Badi S, et al. Neoadjuvant chemotherapy alters neuropilin-1, PlGF, and SNAI1 expression levels and predicts breast cancer patients response. Front Oncol. 2019;9:323–34. https://doi.org/10.3389/fonc.2019.00323.
Grun D, Adhikary G, Eckert RL. NRP-1 interacts with GIPC1 and SYX to activate p38 MAPK signaling and cancer stem cell survival. Mol Carcinog. 2019;58(4):488–99. https://doi.org/10.1002/mc.22943.
Ten Hove T, Van den Blink B, Pronk I, Drillenburg P, Peppelenbosch MP, Van Deventer SJH. Dichotomal role of inhibition of p38 MAPK with SB 203580 in experimental colitis. Gut. 2002;50(4):507–12. https://doi.org/10.1136/gut.50.4.507.
Zubair M, Wang S, Ali N. Advanced approaches to breast cancer classification and diagnosis. Front Pharmacol. 2021;11: 632079. https://doi.org/10.3389/fphar.2020.632079.
Howard FM, Olopade OI. Epidemiology of triple-negative breast cancer: a review. Cancer J. 2021;27(1):8–16. https://doi.org/10.1097/PPO.0000000000000500.
Wu J, Hicks C. Breast cancer type classification using machine learning. J Pers Med. 2021;11(2):61. https://doi.org/10.3390/jpm11020061.
Wu H, Huang JG. PEGylated peptide-based imaging agents for targeted molecular imaging. Curr Protein Pept Sci. 2016;17(6):582–95. https://doi.org/10.2174/1389203717666160101123832.
Sun X, Li Y, Liu T, Li Z, Zhang X, Chen X. Peptide-based imaging agents for cancer detection. Adv Drug Deliv Rev. 2017;110–111:38–51. https://doi.org/10.1016/j.addr.2016.06.007.
Liu Z, Liu S, Wang F, Liu S, Chen X. Noninvasive imaging of tumor integrin expression using 18F-labeled RGD dimer peptide with PEG4 linkers. Eur J Nucl Med Mol Imaging. 2009;36(8):1296–307. https://doi.org/10.1007/s00259-009-1112-2.
Du S, Luo C, Yang G, Gao H, Wang Y, Li X, et al. Developing PEGylated reversed D-peptide as a novel HER2-targeted SPECT imaging probe for breast cancer detection. Bioconjug Chem. 2020;31(8):1971–80. https://doi.org/10.1021/acs.bioconjchem.0c00334.
Sharma AK, Sharma R, Vats K, Sarma HD, Mukherjee A, Das T, et al. Synthesis and comparative evaluation of 177Lu-labeled PEG and non-PEG variant peptides as HER2-targeting probes. Sci Rep. 2022;12(1):15720. https://doi.org/10.1038/s41598-022-19201-9.
Funding
This work was supported by grants from the National Natural Science Foundation of China (22076069, 82202907, U23A20478 and 62171167), Science and Technology Program of Guangzhou (202102020138), Guangdong Basic and Applied Basic Research Foundation (2020A1515011374), Scientific Research Project of Jiangsu Commission of Health (ZD2022036 and M2020028), Development Project of Wuxi (Y20212013) and Wuxi Municipal Health Commission (Q202347). We also acknowledge the financial support received from Jiangsu Provincial Medical Key Laboratory (Key Laboratory of Nuclear Medicine).
Author information
Authors and Affiliations
Contributions
J. Lin, L. Qiu and R. Liu. concepted and designed the project researches; Q. Liu and S. Cai performed theoretical modeling, radiolabeling, cell culture, animal model construction, PET scanning, pharmacokinetics and biodistribution study as well as corresponding data analysis; Q. Liu and Q. Xie performed the western blot, immunofluorescence staining and cellular uptake assay; J. Ye performed the microscale thermophoresis and corresponding data analysis; Q. Liu wrote the manuscript; J. Lin and L. Qiu reviewed and edited the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
This study only involved small animals. All the animal-based experiments were in compliance with the Laboratory Animal Guidelines for the Ethical Review of Animal Welfare of China (No. GB/T 35892–2018) and the Institutional Ethics Board of Jiangsu Institute of Nuclear Medicine.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Q., Cai, S., Ye, J. et al. Preclinical evaluation of 68 Ga-labeled peptide CK2 for PET imaging of NRP-1 expression in vivo. Eur J Nucl Med Mol Imaging (2024). https://doi.org/10.1007/s00259-024-06632-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00259-024-06632-x