Abstract
Evidence of direct reciprocal connections between the cerebellum and basal ganglia has challenged the long-held notion that these structures function independently. While anatomical studies have suggested the presence of cerebellar projections to the substantia nigra pars compacta (SNc), the nature and function of these connections (Cb–SNc) is unknown. Here we show, in mice, that Cb–SNc projections form monosynaptic glutamatergic synapses with dopaminergic and non-dopaminergic neurons in the SNc. Optogenetic activation of Cb–SNc axons in the SNc is associated with increased SNc activity, elevated striatal dopamine levels and increased locomotion. During behavior, Cb–SNc projections are bilaterally activated before ambulation and unilateral lever manipulation. Cb–SNc projections show prominent activation for water reward and higher activation for sweet water, suggesting that the pathway also encodes reward value. Thus, the cerebellum directly, rapidly and effectively modulates basal ganglia dopamine levels and conveys information related to movement initiation, vigor and reward processing.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact K.K.
Code availability
All data reported and the analysis codes used in this study are available upon request.
References
Kelly, R. M. & Strick, P. L. Macro-architecture of basal ganglia loops with the cerebral cortex: use of rabies virus to reveal multisynaptic circuits. Prog. Brain Res. 143, 449–459 (2004).
Kelly, R. M. & Strick, P. L. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J. Neurosci. 23, 8432–8444 (2003).
Alexander, G. E., DeLong, M. R. & Strick, P. L. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 9, 357–381 (1986).
Percheron, G., Francois, C., Talbi, B., Yelnik, J. & Fenelon, G. The primate motor thalamus. Brain Res. Brain Res. Rev. 22, 93–181 (1996).
Person, A. L., Gale, S. D., Farries, M. A. & Perkel, D. J. Organization of the songbird basal ganglia, including area X. J. Comp. Neurol. 508, 840–866 (2008).
Ichinohe, N., Mori, F. & Shoumura, K. A di-synaptic projection from the lateral cerebellar nucleus to the laterodorsal part of the striatum via the central lateral nucleus of the thalamus in the rat. Brain Res. 880, 191–197 (2000).
Hoshi, E., Tremblay, L., Feger, J., Carras, P. L. & Strick, P. L. The cerebellum communicates with the basal ganglia. Nat. Neurosci. 8, 1491–1493 (2005).
Bostan, A. C., Dum, R. P. & Strick, P. L. Cerebellar networks with the cerebral cortex and basal ganglia. Trends Cogn. Sci. 17, 241–254 (2013).
Bostan, A. C., Dum, R. P. & Strick, P. L. The basal ganglia communicate with the cerebellum. Proc. Natl Acad. Sci. USA 107, 8452–8456 (2010).
Chen, C. H., Fremont, R., Arteaga-Bracho, E. E. & Khodakhah, K. Short latency cerebellar modulation of the basal ganglia. Nat. Neurosci. 17, 1767–1775 (2014).
Snider, R. S., Maiti, A. & Snider, S. R. Cerebellar pathways to ventral midbrain and nigra. Exp. Neurol. 53, 714–728 (1976).
Nieoullon, A. & Dusticier, N. Changes in dopamine release in caudate nuclei and substantia nigrae after electrical stimulation of the posterior interposate nucleus of cat cerebellum. Neurosci. Lett. 17, 167–172 (1980).
Nieoullon, A., Cheramy, A. & Glowinski, J. Release of dopamine in both caudate nuclei and both substantia nigrae in response to unilateral stimulation of cerebellar nuclei in the cat. Brain Res. 148, 143–152 (1978).
Carpenter, M. B. Lesions of the fastigial nuclei in the rhesus monkey. Am. J. Anat. 104, 1–33 (1959).
Menegas, W. et al. Dopamine neurons projecting to the posterior striatum form an anatomically distinct subclass. eLife 4, e10032 (2015).
Watabe-Uchida, M., Zhu, L. S., Ogawa, S. K., Vamanrao, A. & Uchida, N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron 74, 858–873 (2012).
Fujita, H., Kodama, T. & du Lac, S. Modular output circuits of the fastigial nucleus for diverse motor and nonmotor functions of the cerebellar vermis. eLife 9, e58613 (2020).
Kwon, H. G. & Jang, S. H. Differences in neural connectivity between the substantia nigra and ventral tegmental area in the human brain. Front. Hum. Neurosci. 8, 41 (2014).
Milardi, D. et al. Extensive direct subcortical cerebellum–basal ganglia connections in human brain as revealed by constrained spherical deconvolution tractography. Front. Neuroanat. 10, 29 (2016).
Thach, W. T. Cerebellar output: properties, synthesis and uses. Brain Res. 40, 89–102 (1972).
Anden, N. E., Hfuxe, K., Hamberger, B. & Hokfelt, T. A quantitative study on the nigro-neostriatal dopamine neuron system in the rat. Acta Physiol. Scand. 67, 306–312 (1966).
Covey, D. P. & Garris, P. A. Using fast-scan cyclic voltammetry to evaluate striatal dopamine release elicited by subthalamic nucleus stimulation. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2009, 3306–3309 (2009).
Nissbrandt, H. & Carlsson, A. Turnover of dopamine and dopamine metabolites in rat brain: comparison between striatum and substantia nigra. J. Neurochem. 49, 959–967 (1987).
Patriarchi, T. et al. Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors. Science 360, eaat4422 (2018).
Chuong, A. S. et al. Noninvasive optical inhibition with a red-shifted microbial rhodopsin. Nat. Neurosci. 17, 1123–1129 (2014).
Lacey, M. G., Mercuri, N. B. & North, R. A. Two cell types in rat substantia nigra zona compacta distinguished by membrane properties and the actions of dopamine and opioids. J. Neurosci. 9, 1233–1241 (1989).
Cho, J. H., Deisseroth, K. & Bolshakov, V. Y. Synaptic encoding of fear extinction in mPFC–amygdala circuits. Neuron 80, 1491–1507 (2013).
Zingg, B., Peng, B., Huang, J., Tao, H. W. & Zhang, L. I. Synaptic specificity and application of anterograde transsynaptic AAV for probing neural circuitry. J. Neurosci. 40, 3250–3267 (2020).
Zingg, B. et al. AAV-mediated anterograde transsynaptic tagging: mapping corticocollicular input-defined neural pathways for defense behaviors. Neuron 93, 33–47 (2017).
Tervo, D. G. et al. A designer AAV variant permits efficient retrograde access to projection neurons. Neuron 92, 372–382 (2016).
Wickersham, I. R. et al. Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron 53, 639–647 (2007).
Reardon, T. R. et al. Rabies virus CVS-N2cΔG strain enhances retrograde synaptic transfer and neuronal viability. Neuron 89, 711–724 (2016).
Boecker, H., Jankowski, J., Ditter, P. & Scheef, L. A role of the basal ganglia and midbrain nuclei for initiation of motor sequences. Neuroimage 39, 1356–1369 (2008).
da Silva, J. A., Tecuapetla, F., Paixao, V. & Costa, R. M. Dopamine neuron activity before action initiation gates and invigorates future movements. Nature 554, 244–248 (2018).
Howe, M. W. & Dombeck, D. A. Rapid signalling in distinct dopaminergic axons during locomotion and reward. Nature 535, 505–510 (2016).
Berardelli, A., Rothwell, J. C., Thompson, P. D. & Hallett, M. Pathophysiology of bradykinesia in Parkinson’s disease. Brain 124, 2131–2146 (2001).
Palmiter, R. D. Dopamine signaling in the dorsal striatum is essential for motivated behaviors: lessons from dopamine-deficient mice. Ann. N.Y. Acad. Sci. 1129, 35–46 (2008).
Panigrahi, B. et al. Dopamine is required for the neural representation and control of movement vigor. Cell 162, 1418–1430 (2015).
Lu, L., Cao, Y., Tokita, K., Heck, D. H. & Boughter, J. D. Jr. Medial cerebellar nuclear projections and activity patterns link cerebellar output to orofacial and respiratory behavior. Front. Neural Circuits 7, 56 (2013).
Legaria, A. A. et al. Fiber photometry in striatum reflects primarily nonsomatic changes in calcium. Nat. Neurosci. 25, 1124–1128 (2022).
Harvey, R. J. & Napper, R. M. Quantitative studies on the mammalian cerebellum. Prog. Neurobiol. 36, 437–463 (1991).
Napper, R. M. & Harvey, R. J. Number of parallel fiber synapses on an individual Purkinje cell in the cerebellum of the rat. J. Comp. Neurol. 274, 168–177 (1988).
Ebner, T. J. & Pasalar, S. Cerebellum predicts the future motor state. Cerebellum 7, 583–588 (2008).
Ito, M. Error detection and representation in the olivo–cerebellar system. Front. Neural Circuits 7, 1 (2013).
Cerminara, N. L., Apps, R. & Marple-Horvat, D. E. An internal model of a moving visual target in the lateral cerebellum. J. Physiol. 587, 429–442 (2009).
Bhanpuri, N. H., Okamura, A. M. & Bastian, A. J. Predicting and correcting ataxia using a model of cerebellar function. Brain 137, 1931–1944 (2014).
Bhanpuri, N. H., Okamura, A. M. & Bastian, A. J. Predictive modeling by the cerebellum improves proprioception. J. Neurosci. 33, 14301–14306 (2013).
Bhanpuri, N. H., Okamura, A. M. & Bastian, A. J. Active force perception depends on cerebellar function. J. Neurophysiol. 107, 1612–1620 (2012).
Therrien, A. S. & Bastian, A. J. Cerebellar damage impairs internal predictions for sensory and motor function. Curr. Opin. Neurobiol. 33, 127–133 (2015).
Honda, T. et al. Tandem internal models execute motor learning in the cerebellum. Proc. Natl Acad. Sci. USA 115, 7428–7433 (2018).
Kawato, M. Internal models for motor control and trajectory planning. Curr. Opin. Neurobiol. 9, 718–727 (1999).
Lisberger, S. G. Internal models of eye movement in the floccular complex of the monkey cerebellum. Neuroscience 162, 763–776 (2009).
Benecke, R., Rothwell, J. C., Dick, J. P., Day, B. L. & Marsden, C. D. Disturbance of sequential movements in patients with Parkinson’s disease. Brain 110, 361–379 (1987).
Pascual-Leone, A., Valls-Sole, J., Brasil-Neto, J. P., Cohen, L. G. & Hallett, M. Akinesia in Parkinson’s disease. I. Shortening of simple reaction time with focal, single-pulse transcranial magnetic stimulation. Neurology 44, 884–891 (1994).
Chen, R., Kumar, S., Garg, R. R. & Lang, A. E. Impairment of motor cortex activation and deactivation in Parkinson’s disease. Clin. Neurophysiol. 112, 600–607 (2001).
Jahanshahi, M., Brown, R. G. & Marsden, C. D. Simple and choice reaction time and the use of advance information for motor preparation in Parkinson’s disease. Brain 115, 539–564 (1992).
Birkmayer, W. & Hornykiewicz, O. The effect of l-3,4-dihydroxyphenylalanine (=DOPA) on akinesia in parkinsonism. Parkinsonism Relat. Disord. 4, 59–60 (1998).
Hornykiewicz, O. The discovery of dopamine deficiency in the parkinsonian brain. J. Neural Transm. Suppl. https://doi.org/10.1007/978-3-211-45295-0_3 (2006).
Hornykiewicz, O. Parkinsonism induced by dopaminergic antagonists. Adv. Neurol. 9, 155–164 (1975).
Ehringer, H. & Hornykiewicz, O. [Distribution of noradrenaline and dopamine (3-hydroxytyramine) in the human brain and their behavior in diseases of the extrapyramidal system]. Klin. Wochenschr. 38, 1236–1239 (1960).
Brown, S. H., Kessler, K. R., Hefter, H., Cooke, J. D. & Freund, H. J. Role of the cerebellum in visuomotor coordination. I. Delayed eye and arm initiation in patients with mild cerebellar ataxia. Exp. Brain Res. 94, 478–488 (1993).
Brunamonti, E. et al. Cerebellar damage impairs executive control and monitoring of movement generation. PLoS ONE 9, e85997 (2014).
Holmes, G. The cerebellum of man. Brain 62, 1–30 (1939).
Holmes, G. The symptom of acute cerebellar injuries due to gunshot wounds. Brain 40, 461–535 (1917).
Trouche, E. & Beaubaton, D. Initiation of a goal-directed movement in the monkey. Role of the cerebellar dentate nucleus. Exp. Brain Res. 40, 311–321 (1980).
Meyer-Lohmann, J., Hore, J. & Brooks, V. B. Cerebellar participation in generation of prompt arm movements. J. Neurophysiol. 40, 1038–1050 (1977).
Miller, A. D. & Brooks, V. B. Parallel pathways for movement initiation of monkeys. Exp. Brain Res. 45, 328–332 (1982).
Tsujimoto, T., Gemba, H. & Sasaki, K. Effect of cooling the dentate nucleus of the cerebellum on hand movement of the monkey. Brain Res. 629, 1–9 (1993).
Heiney, S. A., Kim, J., Augustine, G. J. & Medina, J. F. Precise control of movement kinematics by optogenetic inhibition of Purkinje cell activity. J. Neurosci. 34, 2321–2330 (2014).
Fortier, P. A., Kalaska, J. F. & Smith, A. M. Cerebellar neuronal activity related to whole-arm reaching movements in the monkey. J. Neurophysiol. 62, 198–211 (1989).
Harvey, R. J., Porter, R. & Rawson, J. A. Discharges of intracerebellar nuclear cells in monkeys. J. Physiol. 297, 559–580 (1979).
Thach, W. T. Discharge of cerebellar neurons related to two maintained postures and two prompt movements. I. Nuclear cell output. J. Neurophysiol. 33, 527–536 (1970).
Ohmae, S., Kunimatsu, J. & Tanaka, M. Cerebellar roles in self-timing for sub- and supra-second intervals. J. Neurosci. 37, 3511–3522 (2017).
Thach, W. T. Timing of activity in cerebellar dentate nucleus and cerebral motor cortex during prompt volitional movement. Brain Res. 88, 233–241 (1975).
Mazzoni, P., Hristova, A. & Krakauer, J. W. Why don’t we move faster? Parkinson’s disease, movement vigor, and implicit motivation. J. Neurosci. 27, 7105–7116 (2007).
Heman, P. et al. Nigral degeneration correlates with persistent activation of cerebellar Purkinje cells in MPTP-treated monkeys. Histol. Histopathol. 27, 89–94 (2012).
Necchi, D., Soldani, C., Ronchetti, F., Bernocchi, G. & Scherini, E. MPTP-induced increase in c-Fos- and c-Jun-like immunoreactivity in the monkey cerebellum. Eur. J. Histochem. 48, 385–392 (2004).
Rascol, O. et al. The ipsilateral cerebellar hemisphere is overactive during hand movements in akinetic parkinsonian patients. Brain 120, 103–110 (1997).
Wu, T. & Hallett, M. A functional MRI study of automatic movements in patients with Parkinson’s disease. Brain 128, 2250–2259 (2005).
Cerasa, A. et al. Functional changes in the activity of cerebellum and frontostriatal regions during externally and internally timed movement in Parkinson’s disease. Brain Res. Bull. 71, 259–269 (2006).
Cao, H., Xu, X., Zhao, Y., Long, D. & Zhang, M. Altered brain activation and connectivity in early Parkinson disease tactile perception. AJNR Am. J. Neuroradiol. 32, 1969–1974 (2011).
Martinu, K. & Monchi, O. Cortico–basal ganglia and cortico–cerebellar circuits in Parkinson’s disease: pathophysiology or compensation? Behav. Neurosci. 127, 222–236 (2013).
Tuovinen, N. et al. The reorganization of functional architecture in the early-stages of Parkinson’s disease. Parkinsonism Relat. Disord. 50, 61–68 (2018).
Bologna, M. et al. Effects of cerebellar continuous theta burst stimulation on resting tremor in Parkinson’s disease. Parkinsonism Relat. Disord. 21, 1061–1066 (2015).
Brusa, L. et al. Metabolic changes induced by theta burst stimulation of the cerebellum in dyskinetic Parkinson’s disease patients. Parkinsonism Relat. Disord. 18, 59–62 (2012).
Ferrucci, R. et al. Cerebellar and motor cortical transcranial stimulation decrease levodopa-induced dyskinesias in Parkinson’s disease. Cerebellum 15, 43–47 (2016).
Koch, G. et al. Cerebellar magnetic stimulation decreases levodopa-induced dyskinesias in Parkinson disease. Neurology 73, 113–119 (2009).
Minks, E., Marecek, R., Pavlik, T., Ovesna, P. & Bares, M. Is the cerebellum a potential target for stimulation in Parkinson’s disease? Results of 1-Hz rTMS on upper limb motor tasks. Cerebellum 10, 804–811 (2011).
Teixeira, M. J. et al. Deep brain stimulation of the dentate nucleus improves cerebellar ataxia after cerebellar stroke. Neurology 85, 2075–2076 (2015).
Sokal, P., Rudas, M., Harat, M., Szylberg, L. & Zielinski, P. Deep anterior cerebellar stimulation reduces symptoms of secondary dystonia in patients with cerebral palsy treated due to spasticity. Clin. Neurol. Neurosurg. 135, 62–68 (2015).
Prudente, C. N., Hess, E. J. & Jinnah, H. A. Dystonia as a network disorder: what is the role of the cerebellum? Neuroscience 260, 23–35 (2014).
Ribot, B. et al. Dystonia and dopamine: from phenomenology to pathophysiology. Prog. Neurobiol. 182, 101678 (2019).
Schultz, W. Dopamine reward prediction error coding. Dialogues Clin. Neurosci. 18, 23–32 (2016).
Schultz, W., Apicella, P. & Ljungberg, T. Responses of monkey dopamine neurons to reward and conditioned stimuli during successive steps of learning a delayed response task. J. Neurosci. 13, 900–913 (1993).
Montague, P. R., Dayan, P. & Sejnowski, T. J. A framework for mesencephalic dopamine systems based on predictive Hebbian learning. J. Neurosci. 16, 1936–1947 (1996).
Volkow, N. D. et al. Effects of expectation on the brain metabolic responses to methylphenidate and to its placebo in non-drug abusing subjects. Neuroimage 32, 1782–1792 (2006).
Wagner, M. J., Kim, T. H., Savall, J., Schnitzer, M. J. & Luo, L. Cerebellar granule cells encode the expectation of reward. Nature 544, 96–100 (2017).
Thoma, P., Bellebaum, C., Koch, B., Schwarz, M. & Daum, I. The cerebellum is involved in reward-based reversal learning. Cerebellum 7, 433–443 (2008).
Miquel, M., Gil-Miravet, I. & Guarque-Chabrera, J. The cerebellum on cocaine. Front. Syst. Neurosci. 14, 586574 (2020).
Miquel, M., Nicola, S. M., Gil-Miravet, I., Guarque-Chabrera, J. & Sanchez-Hernandez, A. A working hypothesis for the role of the cerebellum in impulsivity and compulsivity. Front. Behav. Neurosci. 13, 99 (2019).
Miquel, M., Toledo, R., Garcia, L. I., Coria-Avila, G. A. & Manzo, J. Why should we keep the cerebellum in mind when thinking about addiction? Curr. Drug Abuse Rev. 2, 26–40 (2009).
Miquel, M. et al. Have we been ignoring the elephant in the room? Seven arguments for considering the cerebellum as part of addiction circuitry. Neurosci. Biobehav. Rev. 60, 1–11 (2016).
Moulton, E. A., Elman, I., Becerra, L. R., Goldstein, R. Z. & Borsook, D. The cerebellum and addiction: insights gained from neuroimaging research. Addict. Biol. 19, 317–331 (2014).
Guarque-Chabrera, J., Gil-Miravet, I., Olucha-Bordonau, F., Melchor-Eixea, I. & Miquel, M. When the front fails, the rear wins. Cerebellar correlates of prefrontal dysfunction in cocaine-induced memory in male rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 112, 110429 (2022).
Ohmae, S. & Medina, J. F. Climbing fibers encode a temporal-difference prediction error during cerebellar learning in mice. Nat. Neurosci. 18, 1798–1803 (2015).
Heffley, W. et al. Coordinated cerebellar climbing fiber activity signals learned sensorimotor predictions. Nat. Neurosci. 21, 1431–1441 (2018).
Heffley, W. & Hull, C. Classical conditioning drives learned reward prediction signals in climbing fibers across the lateral cerebellum. eLife 8, e46764 (2019).
Kostadinov, D. & Hausser, M. Reward signals in the cerebellum: origins, targets, and functional implications. Neuron 110, 1290–1303 (2022).
Kostadinov, D., Beau, M., Pozo, M. B. & Hausser, M. Predictive and reactive reward signals conveyed by climbing fiber inputs to cerebellar Purkinje cells. Nat. Neurosci. 22, 950–962 (2019).
Larry, N., Yarkoni, M., Lixenberg, A. & Joshua, M. Cerebellar climbing fibers encode expected reward size. eLife 8, e46870 (2019).
Shuster, S. A. et al. The relationship between birth timing, circuit wiring, and physiological response properties of cerebellar granule cells. Proc. Natl Acad. Sci. USA 118, e2101826118 (2021).
Gao, Z. et al. Excitatory cerebellar nucleocortical circuit provides internal amplification during associative conditioning. Neuron 89, 645–657 (2016).
Bina, L., Romano, V., Hoogland, T. M., Bosman, L. W. J. & De Zeeuw, C. I. Purkinje cells translate subjective salience into readiness to act and choice performance. Cell Rep. 37, 110116 (2021).
Carta, I., Chen, C. H., Schott, A. L., Dorizan, S. & Khodakhah, K. Cerebellar modulation of the reward circuitry and social behavior. Science 363, eaav0581 (2019).
Rothermel, M., Brunert, D., Zabawa, C., Diaz-Quesada, M. & Wachowiak, M. Transgene expression in target-defined neuron populations mediated by retrograde infection with adeno-associated viral vectors. J. Neurosci. 33, 15195–15206 (2013).
du Hoffmann, J. & Nicola, S. M. Dopamine invigorates reward seeking by promoting cue-evoked excitation in the nucleus accumbens. J. Neurosci. 34, 14349–14364 (2014).
Gao, Z. et al. A cortico–cerebellar loop for motor planning. Nature 563, 113–116 (2018).
Manto, M. & Oulad Ben Taib, N. Cerebellar nuclei: key roles for strategically located structures. Cerebellum 9, 17–21 (2010).
Lerner, T. N. et al. Intact-brain analyses reveal distinct information carried by SNc dopamine subcircuits. Cell 162, 635–647 (2015).
Beier, K. T. et al. Topological organization of ventral tegmental area connectivity revealed by viral–genetic dissection of input–output relations. Cell Rep. 26, 159–167 (2019).
Franklin, K. & Paxinos, G. The Mouse Brain in Stereotaxic Coordinates (Elsevier, 2008).
Lin, J. Y., Lin, M. Z., Steinbach, P. & Tsien, R. Y. Characterization of engineered channelrhodopsin variants with improved properties and kinetics. Biophys. J. 96, 1803–1814 (2009).
Ting, J. T., Daigle, T. L., Chen, Q. & Feng, G. Acute brain slice methods for adult and aging animals: application of targeted patch clamp analysis and optogenetics. Methods Mol. Biol. 1183, 221–242 (2014).
Berretta, N., Bernardi, G. & Mercuri, N. B. Firing properties and functional connectivity of substantia nigra pars compacta neurones recorded with a multi-electrode array in vitro. J. Physiol. 588, 1719–1735 (2010).
Acknowledgements
The work was funded, in part, by R01MH115604 and R01DA044761 (K.K.). F.N. was supported in part by R01MH060605. We thank the members of the Khodakhah laboratory and S. Nicola for helpful discussions and feedback. dLight used for dopamine measurements was a kind gift from L. Tian. Rabies and helper viruses for retrograde tracing were kind gifts from A. Hantman and K. Ritola. The Zeiss LSM 880 Airyscan confocal microscope of the Neural Cell Engineering and Imaging Core was purchased by funds provided by the NIH Shared Instrument Grant S10OD025295.
Author information
Authors and Affiliations
Contributions
S.W. and R.B. performed in vivo electrophysiology experiments and post hoc histology. S.W. performed in vitro electrophysiological recordings and post hoc histology. M.O. performed anatomical tracing experiments. Image analyses were performed by M.O. and L.K. J.V. performed dopamine measurements and fiber photometry experiments in head-restrained animals on the wheel, with post hoc histology performed by M.O. and J.V. The Pavlovian task and its fiber photometry were performed by J.Y., and post hoc histology was performed by L.K. F.N. supervised and contributed to the analysis of behavioral experiments. K.K. supervised all aspects of the research and secured funding.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks Bernardo Sabatini and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Post-hoc histology of in vivo electrophysiology experiments, related to Fig. 1.
(A) AAV-ChR2-EYPF was injected into the DCN and an optrode was placed in the SNc for in vivo recording. (B) (Top, left) Schematic of SNc region zoom in. (Top, right) Expression of ChR2 in cerebellar fibers in SNc was visualized with YFP (green). TH staining was performed to delimit the structure and to label for dopaminergic neurons (blue). Scale bar: 250 µm. (Bottom) Different examples showing cerebellar axons in SNc. Scale bars: 100 µm. (C) (Top) Example of the lesion in the tissue caused by the optrode in the SNc. (Bottom) To track the position of the optrode, it was labeled with DiI (red). Scale bars: 250 µm.
Extended Data Fig. 2 Increasing the strength of optogenetic activation of Cb-SNc axons in vivo increases the response of SNc neurons, related to Fig. 1.
(A) The response of SNc neurons increased with increasing laser intensities. Example response of a neuron to increasing intensities of light stimulation. (B) Summary of cells recorded from SNc under stimulation with various light intensities.
Extended Data Fig. 3 Post-hoc histology of dopamine measurements experiments, related to Fig. 1.
(A) AAV-ChR2-EYFP was injected in the DCN and AAV-dlight1.1 in the striatum. Optic fibers were implanted in the SNc to stimulate Cb-SNc axons and in the striatum to measure dopamine fluctuations. (B) (Left) Schematic of SNc region zoom in. (Right) Optic fiber location in SNc. Scale bar: 250 µm. (C) (Left) Schematic of striatum region zoom in. (Right) Optic fiber location in striatum. Scale bar: 1 mm.
Extended Data Fig. 4 Raw data and onset latency of striatal dopamine evoked by Cb-SNc activation, related to Fig. 1j,k.
(A) Mice were injected with ChR2 in the DCN, and the dopamine sensor dLight1.1 in the striatum. Optic fibers were implanted in SNc to deliver optogenetic pulses and in the dorsolateral striatum to monitor dopamine fluctuations. (B) Raw data traces of dopamine dLight1.1 signals recorded in the dorsolateral striatum. Both spontaneous and optogenetically-evoked (single 1 ms pulses, top or 20 Hz trains, bottom) events are present. (C) (Left) Dopamine signal Z-score aligned to the time of optogenetic stimulation (1 ms pulse at 1 mW, mean ± SEM, n = 7 fibers, N = 4 mice). (Right) Expansion of the first data point after optogenetic stimulation (dotted box). Open circles are the mean Z-score of all the trials (128) in each mouse. One-sided paired t-test of the mean data points obtained just before and just after optogenetic stimulation.
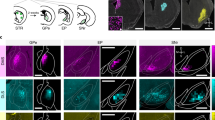
Extended Data Fig. 5 Anatomical evidence for monosynaptic projections from the DCN to the SNc, related to Fig. 3.
(A) Experimental design. RCE:loxp mice were injected bilaterally with AAV1.Cre to label DCN-recipient cells in the SNc. (B) Representative image of AAV1-Cre transfected neurons in the three DCN. DN: dentate nucleus, IntN: interposed nucleus, FN: fastigial nucleus, VN: vestibular nucleus. Scale bar: 250 µm. (C) Average heatmap of the DCN AAV1-Cre transfection efficacy (N = 3). Scale bar: 250 µm. (D) Representative image of the SNc. TH positive neurons are labeled in red, SNc neurons receiving inputs from the DCN are labeled with GFP in green, nuclei are labeled with DAPI in blue. Both TH-positive (arrow) and TH-negative (arrowhead) SNc neurons were targeted by the DCN. Scale bars: 50 and 25 µm. (E) Locations of all DCN-targeted neurons along the SNc. N = 3 mice. Scale bar: 200 µm. (F) Number of DCN-targeted neurons in the SNc along the antero-posterior axis. Neuronal counts from both hemispheres in each section of the SNc were combined. Mean ± SEM, N = 3.
Extended Data Fig. 6 Post-hoc histology of dopamine measurements in DLS, related to Fig. 5.
(A) AAV-ChR2-EYFP was injected in the DCN and AAV-dlight1.1 in the striatum. Optic fibers were implanted in the SNc to stimulate Cb-SNc axons and in the striatum to measure dopamine fluctuations. (B) (Left) Schematic of SNc region zoom in. (Right) Optic fiber location in SNc.ChR2 is shown in green (YFP), TH in red and nuclei is blue (DAPI). Scale bar: 200 µm. (C) (Left) Schematic of striatum region zoom in. (Right) Optic fiber location in dorsolateral striatum. Scale bar: 200 µm.
Extended Data Fig. 7 Post-hoc histology of simultaneous fiber photometry recordings of Cb-SNc axons and SNc neurons in mice on the wheel, related to Fig. 6a–c.
(A) AAV-GCaMP7 was injected in the DCN and AAV-jRGECO1 was injected in the SNc. Optic fiber was implanted in the SNc for dual recordings. (B) Schematic of SNc region. (C) (Left) Expression of jRGECO and fiber location in SNc. Scale bar: 1 mm. (Right) A zoom in of the image shows jRGECO neurons in red. TH was used to delimit SNc area (blue). Optic fiber was located above the SNc. Scale bar: 250 µm.
Extended Data Fig. 8 Post-hoc histology of single fiber photometry recordings in mice performing the lever-manipulation and Pavlovian tasks, related to Fig. 7d,e and 7a–c.
(A) AAV-GCaMP7 was injected in the DCN. Optic fibers were implanted in the SNc to measure the Cb-SNc activity. (B) (Left) Schematic of SNc region. (Right) Optic fiber location in SNc. Scale bar: 1 mm.
Extended Data Fig. 9 Reward-related activity of cerebellar axons in the SNc is bilateral, related to Fig. 7a,b.
(A) Schematic of bilateral recording of Cb-SNc axons during regular reward consumption. (B) GCaMP signals from Cb-SNc axons recorded simultaneously from the right (top) and the left (bottom) SNc during regular reward trials. Mean ± SEM.
Extended Data Fig. 10 Post-hoc histology of dual fiber photometry recordings in mice performing a Pavlovian task, related to Fig. 7c–f.
(A) AAV-GCaMP7 was injected in the DCN and AAV-FLEX-jRGECO1 in the SNc of DAT-Cre mice. An optic fiber was implanted in the SNc to measure Cb-SNc axons and SNc-DA neurons activity while an optic fiber in the dorsolateral striatum (DLS) recorded SNc-DA neurons axons. (B) Schematic of SNc region (zoom in). Expression of jRGECO and fiber location in SNc. jRGECO neurons (in red) colocalized with TH (blue) and Cb-SNc fibers expressing GCaMP (green). Optic fiber was located above the SNc. Scale bar: 250 µm. (C) Schematic of striatum (zoom in). Optic fiber location in DLS. Scale bar: 1 mm.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Washburn, S., Oñate, M., Yoshida, J. et al. The cerebellum directly modulates the substantia nigra dopaminergic activity. Nat Neurosci 27, 497–513 (2024). https://doi.org/10.1038/s41593-023-01560-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-023-01560-9