Abstract
Anticoagulants are widely used as potent cumulative rodenticides; however, they are also associated with adverse environmental effects, such as intoxication of non-target animals. To ensure user safety, in the EU the use of anticoagulants containing active substances of > 30 ppm is restricted to professionals as these substances are toxic to reproduction Kat.1 A or B. Therefore, new products with < 30 ppm anticoagulant substance have been developed and registered. While the biological efficacy of these new products has been evaluated, the effect of their application on liver residues in targeted rodents was not examined before product introduction to the market. Our laboratory no-choice study on wild brown rats (Rattus norvegicus) showed that baits with high (50 ppm) and low (25 ppm) brodifacoum concentrations (with identical nontoxic cereal-based food components) presented 100% efficacy, although the 25 ppm bait exhibited significantly higher palatability and 4.5 times higher liver residues. Rats consumed 13.6 and 167.7 g of the 50 and 25 ppm baits, respectively, which corresponded to 3.16 and 14.91 μg/g of brodifacoum liver residues at their time of death. The experiments demonstrated that a decreased concentration of brodifacoum anticoagulant in rodenticide baits may lead to greater accumulation in rodent liver, thus indicating the urgent need to perform a detailed study of the environmental risks of low-concentration anticoagulant baits.
Similar content being viewed by others
Key Message
-
Varying concentration of anticoagulants are used in rodenticide baits since 2018
-
Low brodifacoum concentration in baits resulted in high palatability in brown rats
-
Greater quantity of ingested bait resulted in 4.5 x higher liver brodifacoum residue
-
Decreased concentration of brodifacoum in baits leads to increased liver residues
Introduction
Synanthropic rodents of the genus Rattus (Muridae) are devastating pests in urban, rural and natural environments, and are distributed nearly worldwide. They damage agricultural crops, commodities and processed feed and food by feeding/gnawing and contaminating with faeces and foul-smelling urine. Recently, the World Health Organization (WHO) confirmed synanthropic rodents to be a significant public and veterinary health threat worldwide due to the transmission of pathogens, such as bacteria and viruses (WHO 2019). As invasive predatory animals, synanthropic rodents also represent direct threats to native species after their penetration into closed island ecosystems (Towns et al. 2006; Wheeler et al. 2019). To mitigate such pest risks, anticoagulant rodenticides (ARs) in the form of baits are frequently used to regulate or exterminate rodent populations. However, their use is associated with unintended poisoning through primary (direct bait consumption) and secondary (consumption of an intoxicated individual) exposure of non-target organisms (i.e., wild and farm animals, and household pets). There has been increasing evidence of primary and secondary intoxication of non-target wildlife or the sublethal presence of anticoagulant residues in small mammals, birds, bird and mammalian predators and scavengers during last decades (e.g., Sánchez-Barbudo et al. 2012; Geduhn et al. 2014; Walther et al. 2021a). In particular, more potent second-generation ARs persist in animal bodies and are thus dangerous. ARs bioaccumulate in liver and persists there for dozens (first-generation ARs) or hundreds (second-generation ARs) of days (Horak et al. 2018). Thus, AR in the liver of both target and non-target species pose significant environmental risks via secondary exposure.
With the regulation (EU) 2016/1179, the anticoagulant rodenticides containing an active substance above 30 ppm were classified as toxic to reproduction Kat.1 A or B. According to article 19 (4) a) of the Biocidal Products Regulation (BPR) 528/2012/EU such classed biocidal products cannot be used by the general public. This is to ensure user safety. Therefore, the manufacturer of rodenticides enforced the development of products with lower (< 30 ppm, i.e., nearly half as 50 ppm was common) anticoagulant concentrations in some rodenticide products since 2018, so that the products can continue to be sold to the consumer. Efficacy of new low-concentration products has been demonstrated for several low concentration products. Furthermore, during the approval process, it is assumed, that beside the lower user risk a low (< 30 ppm) active substance concentration results also in consequent lower ingested anticoagulant dosages and lower anticoagulant liver residues in poisoned rodents. Such theoretical reasoning appears logical for the mitigation of adverse environmental effects, with the lower amount of anticoagulant in baits leading to a decrease in the risk of primary and secondary intoxication. However, this seemingly logical assumption regarding bait consumption was not experimentally evaluated or the results of such a validation have not been published or are not publicly available.
In our previous preliminary tests (unpublished data) with various brodifacoum-based baits on brown rats (Rattus norvegicus) we observed substantial consumption of baits with a lower active substance content before the rat’s death compared to baits with a higher active substance content. Based on preliminary observations, we hypothesized that the higher consumption of low-concentration bait may result in higher brodifacoum bioaccumulation in liver. To test this hypothesis, experiments with a R. norvegicus wild strain were designed and performed under controlled laboratory conditions: (i) The first objective was to compare the efficacy (mortality and time to death) and intake of baits with high and low AR concentrations. (ii) The second objective was to determine the accumulated level of AR residues in the liver of the tested individuals for baits with high and low anticoagulant concentrations at the time of rodent death.
Material and methods
Animals
Brown rats (R. norvegicus) were descendants of wild individuals trapped in the Central Bohemian region (Czech Republic). These rats were third and fourth generation progeny born in captivity. Rats were maintained in family groups under standard laboratory conditions (temperature 19–22 °C, 12:12 light cycle) in wire-mesh cages (260 × 450 × 260 mm) resistant to gnawing. Wood shavings serving as bedding material, and water and food (ST1; Velaz, Ltd., Czech Republic, occasionally supplemented with dry bread and apples) were provided ad libitum.
Testing protocol
In this initial work, we tested the effects of the bait products with high and low concentrations of brodifacoum that are already on the market, i.e., the baits have passed all the regulatory approval tests/procedures necessary for authorization of biocide products in the EU according to the BPR. The experiments were conducted within our research activities on anticoagulant rodenticides and not as any approval or authorization tests. Therefore, the experimental protocol did not follow an official European guideline, but was designed as an original research procedure that is described below.
No-choice feeding tests
We tested 24 individuals, 9 males (mean body weight = 362 g, range 268–462 g) and 15 females (mean body weight = 265 g, range 198–319 g). At the beginning of the trials, the rats were housed individually in the same sized cages as the breeding ones, and after the 3-day acclimatization period, the trial was initiated. To assess the daily food intake as control values, 11 randomly assigned individuals (3 males, 8 females) were monitored for three days (further referred as control days) and the daily consumption of standard laboratory diet (ST1) was recorded. Subsequently, laboratory diet was removed from the cages and the experimental phase began (further referred as experimental days). During this phase, all 24 animals were fed an AR bait (Norat ATG; PelGar, Ltd., Czech Republic). Two commercial brodifacoum pellet baits with identical cereal-based food components that differed only in the concentration of the active ingredient brodifacoum were tested. A high concentration of 0.05 g/kg (50 ppm) in 3 males (mean body weight = 441 g, range 421–462 g) and 9 females (mean body weight = 276 g, range 215–319 g) and a low concentration of 0.025 g/kg (25 ppm) in 6 males (mean body weight = 322 g, range 268–391 g) and 6 females (mean body weight = 249 g, range 198–292 g). The balanced sex ratio design for the low-concentration bait was favored because of no published data on efficacy and bait intake of these new products.
Laboratory diet or baits and water were provided ad libitum during the trials. Rats were examined daily, and the remaining diet (control values) or baits (experimental values) were weighed (including spillage and crumbled food) and replenished. These procedures were repeated daily, and the mortality was recorded. The experiment ran to death in all animals, signs of suffering (reduced activity/hunched posture, external bleeding) were recorded. Animal carcasses were stored at − 20 °C until further analysis.
To confirm the declared concentration of brodifacoum in the baits (claimed by the producer on the label), the specific concentration of brodifacoum in both baits was performed by high performance liquid chromatography (HPLC). The analysis was conducted in the producer´s analytical chemistry laboratory (Agrochema druzstvo, Studenec) according to their standard procedures based on the SANCO/3030/99 rev. 4 guidelines (EC 2000). Pellets were ground to a fine powder and mixed to create a homogenous matrix. A 2.5 g subsample was weighed in a 50 ml volumetric flask and partially made to volume using methanol. The mixture was placed in an ultrasonic bath and sonicated for 20 min, cooled and diluted in methanol. The supernatant liquid was used for the HPLC-UV (Agilent HPLC-UV 1100 series) and LC–MS Q-ToF analysis (Agilent 1200 series HPLC-DAD, Agilent 6500 series Q-ToF Mass Spectrometer). Mean recovery was 101.2%, sample precision 1.75% and the limit of detections 0.92 ppm.
The measured concentrations were 0.00506 and 0.00240% for the high (50 ppm) and low (25 ppm) nominal concentrations, respectively. These measured values were used to calculate the total dosages ingested by individual rats.
Brodifacoum residues in liver of rats
Rat carcasses were transferred to the Chemistry department of State Veterinary Institute, Prague; the laboratory is certified for toxicological analyses detecting residues of pesticides (including anticoagulants). Only 21 rats were transferred for the analysis as three individuals (2 females, 1 male) fed the 50 ppm bait were accidentally disposed of by staff during the storage. The liver tissue samples were collected and extracted by Mini-Multiresidue Method QuEChERS. The tissue was homogenized, a sample of 10 g was transferred to a 50 ml tube and 3 ml water was added. Next, internal standards, acetonitrile and extraction salts were added, shaken and centrifuged. The supernatant liquid was used for the LC–MS/MS (Dionex UltiMate 3000 RS Series, AB Sciex 5500 QTRAP). Mean recovery was 98.5%, sample precision 8.5% and the limit of detections 0.002 ppm.
Statistical analysis
Firstly, survival times, total bait intake and brodifacoum dosage ingestion were evaluated by analysis of variance, where the effects of sex, bait type and on sex*bait type interaction were examined.
For further comparison, the individual bait consumption was expressed as the amount of diet/bait consumed per day and per kg of individual body weight (bw). Differences in consumption during control phase and experimental phases with two baits were tested using analysis of variance with post hoc comparisons using Tukey HSD method. Corresponding brodifacoum intake (mg/kg rat) was compared between two baits by t-test.
As brodifacoum liver residues between high and low concentrations baits assessed in samples with unequal size, Welch’s t-test for unequal variances was used for comparison.
All calculations were performed using Statistica 13.3 (TIBCO Software Inc. 2017).
Results
Survival times
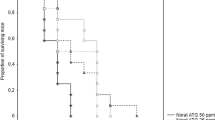
All brown rats readily accepted the baits with both concentrations of brodifacoum, and 100% of them died within 21 days. The mean survival (± SD) was 7.8 ± 1.7 and 10.7 ± 5.5 days for rats fed 50 and 25 ppm, respectively. Survival times did not differ between sexes (F(1,20) = 14.3, p > 0.05), bait types (F(1,20) = 46.3, p > 0.05) and also sex*bait type interaction (F(1,20) = 5.1, p > 0.05) remained nonsignificant. Several rats fed the 25 ppm bait survived longer than those fed the 50 ppm bait (Fig. 1), but the times to death were not statistically significant.
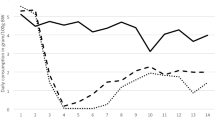
Mean daily consumption (means ± SD) and survival of rats exposed to brodifacoum based baits (50 and 25 ppm) in the no-choice feeding test. C1–C3 represent the control values when rats were fed a laboratory diet (n = 5 for 50 ppm group, dotted black bars; n = 6 for 25 ppm group, dotted white bars); E1–E21 represent the experimental days (n = 12 for 50 ppm group, black bars; n = 12 for 25 ppm group, white bars)
During the experiment, changes in the appearance or behavior were recorded in both tested groups. These changes were observed in 9 individuals from each of the tested group, the rest of animals (6 in total) exhibited no such signs of intoxication. Rats fed the 50 ppm bait displayed reduced activity/hunched posture in 8 individuals (started from day 5, 5, 6, 6, 6, 7, 8, 9 of the experiment) and external bleeding only in one rat (started from day 7 of the experiment). Rats fed the 25 ppm bait exhibited predominantly external bleeding; it was recorded in 8 individuals (started from day 2, 4, 7, 7, 7, 7, 9, 16 of the experiment). One rat displayed only reduced activity/hunched posture, this behavior was recorded from the 8th day of the experiment.
Bait intake and brodifacoum liver residues
The average total bait consumption was 13.6 ± 4.0 and 166.0 ± 113.8 g per rat for the 50 and 25 ppm bait, respectively. There was a significant effect of bait type (F(1,20) = 18.3, p < 0.001) on total consumption; whereas, the sex (F(1,20) = 0.8, p > 0.05) and sex*bait type interaction (F(1,20) = 0.8, p > 0.05) had no effect on the bait intake.
Rats fed the high and low concentration baits differed significantly in the amount of total ingested brodifacoum (F(1,20) = 14.7, p = 0.001), for means see Table 1. The factor sex (F(1,20) = 0.8, p > 0.05) and sex*bait type interaction (F(1,20) = 0.9, p > 0.05) did not affect the intake.
As rats fed the 50 ppm bait consumed a significantly lower amount of the bait (Fig. 1), bait intake during the first exposure day was further evaluated to determine if the sufficient amount of the 50 ppm bait, i.e., lethal dose of brodifacoum LD50, was ingested in this experimental group. Compared with the published acute oral median lethal dose of brodifacoum for R. norvegicus (LD50 = 0.26–0.56 mg/kg; Redfern et al. 1976; Laakso et al. 2010), the experimental rats consumed 1.1–4.9 times of the bait to ingest LD50.
As the individual rats were exposed for different durations (mortality range 3–21 days) and differed in body weight, the individual bait consumption expressed per day and per kg of individual body weight (bw) was used for further comparison. Rats consumed different amounts of diet/bait per day and kg (bw) during the trial (F(2,29) = 93.4, p < 0.001). There was a non-significant trend regarding the factor of sex (F(1,29) = 4.1, p = 0.05; mean for females = 48.28 g/kg, mean for males = 40.16 g/kg); sex*bait type interaction (F(2,29) = 2.1, p > 0.05) did not affect the intake. The post hoc tests revealed that rats fed the 50 ppm bait ingested significantly less bait than those fed 25 ppm bait and also than rats during the control phase (both p = 0.001). At the same time, rats fed the 25 ppm bait ingested significantly less bait than those of the control group (p < 0.005); for means, see Table 1.
Rats fed the 50 ppm bait ingested significantly less of the corresponding brodifacoum dosage per day and per kg of bw than those fed the 25 ppm bait (F(1,20) = 14.2, p = 0.001). The factor sex (F(1,20) = 2.1, p > 0.05) and sex*bait type interaction (F(1,20) = 1.4, p > 0.05) did not affect the brodifacoum dosage; for means see Table 1.
Because of highly variable data on bait and brodifacoum intake a raw data table for each animal can be found as Supplementary Table S1 online.
The brodifacoum residues differed significantly between the tested groups (t = 6.33, p < 0.001; Fig. 2). The mean liver concentrations of brodifacoum were 3.16 ± 1.34 and 14.91 ± 6.23 μg/g for the 50 and 25 ppm bait, respectively.
Discussion
The recently adopted regulation (EU) 2016/1179 ensuring user safety resulted to the development of ARs with lower concentration of active substances. Our no-choice study describes a phenomenon of concern in which a decrease in the anticoagulant active substance in rodenticide baits may actually result in higher residues of ARs in rodents and thus to an increase in the potential environmental toxic load caused by use of the low-concentration bait. The mean liver concentration of brodifacoum after consumption of the 25 ppm bait was 4.5 × greater than that after consumption of the 50 ppm bait. Indeed, all rats fed the 25 ppm bait had higher liver residues than those fed the 50 ppm bait. Brodifacoum is one of the most potent AR substances in rodenticide baits. Its persistence in liver tissues of both target and non-target animals is extremely high, and the hepatic half-life in rats was estimated to be 130–350 days (Fisher et al. 2003; Erickson and Urban 2004). Thus, among other anticoagulants, brodifacoum presents a high risk for secondary poisoning in predators and scavengers (Fisher et al. 2004) as evidenced by frequent detection in liver (e.g. Fourel et al. 2021; Nakayama et al. 2019). In rats, brodifacoum liver residues after consumption of low concentration bait (20 or 25 ppm) have been assessed by several authors, and data are available from laboratory experiments (residues = 10.7 μg/g: Fisher et al. 2004) and field conditions (residues = 18.86, 6.01 μg/g: Pitt et al. 2015; Walther et al. 2021b). These values correspond well with our results for the 25 ppm bait (14.9 μg/g). Higher liver residues in rats fed the low-concentration bait were a consequence of both the higher palatability of low-concentration bait and the prolonged survival of individuals fed those baits. Our testing approach and conditions, which involved multiple-day no-choice feeding, represent an extreme scenario for rodenticide intake. This scenario assumes that no alternative food sources are available, leaving the experimental rats with no option but to consume the brodifacoum baits. Therefore, it is difficult to transfer the data to the situation under real conditions, since there the animals usually have alternative feed available and interactions between the animals take place. However, the laboratory scenario simplifies interpretation and focus solely on the effect of two factors, the concentration of brodifacoum (50 and 25 ppm) without considering interactions with other factors such as alternative food. Furthermore, our recent study has demonstrated that synanthropic rodents find various commercial rodenticide formulations highly palatable, with little difference observed between choice and no-choice tests (Frankova et al. 2022). Nevertheless, to expand the present results and make more general conclusions, future research should include field tests under real conditions or choice tests with various alternative food sources to simulate the field conditions with poor sanitation or environments abundant in alternative nutrient supply (e.g., animal farms, grain stores, agricultural premises, garbage sites, communal waste, etc.).
High palatability of the low concentration bait was previously demonstrated in food choice studies with rats (Fisher et al. 2004) and food no-choice trials with house mice (Mus musculus) (Frankova et al. 2019). The latter study on mice (Frankova et al. 2019) revealed similar differences in bait consumption between the high- and low-concentration (50 and 25 ppm) baits (i.e., mice consumed considerably more of the 25 ppm bait). Because identical bait products (Norat ATG) were tested in this cited study and in the present study, we conclude that the decrease in active substances (brodifacoum) led to increased bait intake in both rodent species, and this variance is seemingly more noticeable in rats. Under field conditions, rats may repeatedly visit palatable low-concentration baits and consume them in greater amounts compared to high-concentration baits, which subsequently results in a greater brodifacoum load in rat bodies, as suggested by Walther et al. (2021b). Based on their results, Walther et al. (2021b) hypothesized that large brodifacoum doses during field application could lead to rapid death, which could balance the high brodifacoum intake. However, our laboratory data do not support this hypothesis. All the exposed rats died during the trial; thus, we confirmed the high efficacy of both baits tested. Nevertheless, survival time in the 50 ppm group could be affected in some way by food avoidance/starvation as low bait intake was found in this bait. However, it must be stressed that all of the 50 ppm tested rats consumed 1.1–4.9 times of the bait to ingest LD50 of brodifacoum right on the first exposure day. The low consumption and sharp decline of consumption on the 50 ppm bait was not quite surprising to us since we previously observed this phenomenon in mice too (Frankova et al. 2019). In addition, in the different study Frankova et al. (2017) observed that although visible intoxication came with the delay of several days, the food (brodifacoum based bait) consumption was rapidly suppressed right from the first day. However, in the previous mice studies (i.e., Frankova et al. 2017, 2019) bioaccumulation of brodifacoum in mice livers were not estimate unlike in the present rat study. Despite substantial intake of the 25 ppm bait (and corresponding higher brodifacoum dosage) from the first experimental day, rats consuming this bait tended to survive longer (this tendency was not statistically significant). As only half of the low concentration group was dead after 10 days, it seems that the low concentration bait is less efficient (some individuals had lower sensitivity to brodifacoum), although ingestion of higher brodifacoum concentration from the first day. We have no particular explanation for this observed effect associated the low concentration bait. However, prolonged survival on brodifacoum-based bait was also previously detected in study with house mice (Wheeler et al. 2019; Cuthbert et al. 2011). Moreover, the aforementioned study of Frankova et al. (2019), which tested the same bait products (Norat ATG, 25 and 50 ppm) on mice, is coincident with the present study as in both studies rodents fed the 25 ppm bait ingested multiple times of bait than those fed the 50 ppm bait. While the 25 ppm bait intake at the first four days of the trial corresponds well with food intake during the control period, consumption of the 50 ppm bait was substantially decreased. The specific mechanism at a deeper physiological, toxicological, pharmacokinetic or ethological level, responsible for such low palatability remains unclear. Under real condition, both bait consumption and survival is affected by social interaction, e.g., higher potential for injuries under real conditions (rank fights) leading to faster bleeding of the animals and different feeding pattern of different sex or population (Inglis et al. 1996; Klemann and Pelz 2006).
There is a tendency in the EU to reduce the concentration of active ingredients of rodenticides in the baits for non-professional use for human health (user) safety. This work found that tested bait with a reduced brodifacoum concentration was associated with a trend to slower rodent mortality, while paradoxically leading to a higher accumulation of the rodenticide in the rodent liver than was the case for a bait with a higher brodifacoum concentration. Here, it should be noted that the paper only describes the phenomenon of increased accumulation of the anticoagulant that we documented for the approved and commercially marketed product with reduced concentration. Our work did not aim to determine the toxicological and physiological mechanisms of the paradoxical phenomenon observed. Thus, this was not a manipulative experiment working with laboratory-prepared baits. However, the work is valuable from the practical point of view: it highlights the risk that realistic use of a given registered product may result in increased accumulation of the anticoagulant in the liver of affected rodents, which may have a negative effect on non-target species (scavenger predators). It should be noted that the study was performed on one particular preparation. Thus, verification is needed whether the physiological and toxicological activity of other preparations (and active ingredients) act in a similar way, and therefore enables generalization of our result. Furthermore, data should be collected under real conditions to determine whether the practical application of a bait with a reduced active ingredient content also leads to higher residues in the target organisms. In conclusion, our results show that the development of bait with reduced concentrations intended for the general public may lead to unfortunately increased accumulation of the toxicant in rodent liver and thus, may have adverse environmental consequences.
Author contributions
MF, RA and VS conceived and designed research. MF and TR conducted experiments. MF and TR analyzed data. MF and VS wrote the manuscript. All authors reviewed the manuscript.
Data availability
The data are available from the corresponding author on reasonable request.
References
Cuthbert RJ, Visser P, Louw H, Ryan PG (2011) Palatability and efficacy of rodent baits for eradicating house mice (Mus musculus) from Gough Island, Tristan da Cunha. Wildl Res 38:196–203. https://doi.org/10.1071/WR11016
Erickson W, Urban D (2004) Potential risks of nine rodenticides to birds and nontarget mammals: a comparative approach. US Environmental Protection Agency, Washington, p 225
European Commission (2000) Technical material and preparations: guidance for generating and reporting methods of analysis in support of pre‐ and post‐registration data requirements for Annex II (Part A, Section 4) and Annex III (Part A, Section 5) of Directive 91/414. SANCO/3030/99‐rev. 4. Accessed 11 July 2000
Fisher P, O’Connor C, Wright G, Eason CT (2003) Persistence of four anticoagulant rodenticides in the livers of laboratory rats. DOC Sci Intern Ser 139:19
Fisher P, O’Connor C, Wright G, Eason CT (2004) Anticoagulant residues in rats and secondary non-target risk. DOC Sci Intern Ser 188:29
Fourel I, Couzi FX, Lattard V (2021) Monitoring the hepatic residues of cis- and trans-diastereoisomers of second generation anticoagulant rodenticides reveals a different bioaccumulation of diastereoisomers in the food chain of the réunion harrier (Circus maillardi). Sci Total Environ 779:146287. https://doi.org/10.1016/j.scitotenv.2021.146287
Frankova M, Stejskal V, Aulicky R (2017) Suppression of food intake by house mouse (Mus musculus) following ingestion of brodifacoum-based rodenticide bait. Crop Prot 100:134–137. https://doi.org/10.1016/j.cropro.2017.06.017
Frankova M, Stejskal V, Aulicky R (2019) Efficacy of rodenticide baits with decreased concentrations of brodifacoum: validation of the impact of the new EU anticoagulant regulation. Sci Rep 9:16779. https://doi.org/10.1038/s41598-019-53299-8
Frankova M, Aulicky R, Stejskal V (2022) Efficacy of eight anticoagulant food baits in house mouse (Mus musculus): comparison of choice and no-choice laboratory testing approaches. Agronomy 12:1828. https://doi.org/10.3390/agronomy12081828
Geduhn A, Esther A, Schenke D, Mattes H, Jacob J (2014) Spatial and temporal exposure patterns in non-target small mammals during brodifacoum rat control. Sci Total Environ 496:328–338. https://doi.org/10.1016/j.scitotenv.2014.07.049
Horak KE, Fisher PM, Hopkins B (2018) Pharmacokinetics of anticoagulant rodenticides in target and non-target organisms. In: Van den Brink N et al (eds) Anticoagulant Rodenticides and Wildlife. Springer, Cham, pp 87–108. https://doi.org/10.1007/978-3-319-64377-9_4
Inglis IR, Shepherd DS, Smith P, Haynes PJ, Bull DS, Cowan DP, Whitehead D (1996) Foraging behaviour of wild rats (Rattus norvegicus) towards new foods and bait containers. Appl Anim Behav Sci 47:175–190. https://doi.org/10.1016/0168-1591(95)00674-5
Klemann N, Pelz HJ (2006) The feeding pattern of the Norway rat (Rattus norvegicus) in two differently structured habitats on a farm. Appl Anim Behav Sci 97:293–302. https://doi.org/10.1016/j.applanim.2005.08.004
Laakso S, Suomalainen K, Koivisto S (2010) Literature review on residues of anticoagulant rodenticides in non-target animals. TemaNord, Copenhagen. https://doi.org/10.6027/TN2010-541
Nakayama SMM, Morita A, Ikenaka Y, Mizukawa H, Ishizuka M (2019) A review: poisoning by anticoagulant rodenticides in non-target animals globally. J Vet Med Sci 81:298. https://doi.org/10.1292/jvms.17-0717
Pitt WC, Berentsen AR, Shiels AB, Volker SF, Eisemann JD, Wegmann AS, Howald GR (2015) Non-target species mortality and the measurement of brodifacoum rodenticide residues after a rat eradication on Palmyra Atoll, tropical Pacific. Biol Conserv 185:36–46. https://doi.org/10.1016/j.biocon.2015.01.008
Redfern R, Gill JE, Hadler MR (1976) Laboratory evaluation of WBA 8119 as a rodenticide for use against warfarin-resistant and non-resistant rats and mice. Epidemiol Infec 77:419–426. https://doi.org/10.1017/s0022172400055807
Sánchez-Barbudo IS, Camarero PR, Mateo R (2012) Primary and secondary poisoning by anticoagulant rodenticides of non-target animals in Spain. Sci Total Environ 420:280–288. https://doi.org/10.1016/j.scitotenv.2012.01.028
Towns DR, Atkinson IAE, Daugherty CH (2006) Have the harmful effects of introduced rats on islands been exaggerated? Biol Invasions 8:863–891. https://doi.org/10.1007/s10530-005-0421-z
Walther B, Ennen H, Geduhn A, Schlötelburg A, Klemann N, Endepols S, Schenke D, Jacob J (2021a) Effects of anticoagulant rodenticide poisoning on spatial behavior of farm dwelling Norway rats. Sci Total Environ 787:147520. https://doi.org/10.1016/j.scitotenv.2021.147520
Walther B, Geduhn A, Schenke D, Schloetelburg A, Jacob J (2021b) Baiting location affects anticoagulant rodenticide exposure of non-target small mammals on farms. Pest Manag Sci 77:611–619. https://doi.org/10.1002/ps.5987
Wheeler R, Priddel D, O’Dwyer T, Carlile N, Portelli D, Wilkinson I (2019) Evaluating the susceptibility of invasive black rats (Rattus rattus) and house mice (Mus musculus) to brodifacoum as a prelude to rodent eradication on Lord Howe Island. Biol Invasions 21:833–845. https://doi.org/10.1007/s10530-018-1863-4
WHO (2019) Expert meeting on “innovative control approaches of rodent-borne epidemic diseases and other public health consequences of rodents’ proliferation”. WHO Meeting Report. https://ecorodman.nri.org/images/WHO_rodent_meeting.pdf. Accessed 13 Oct 2021
Acknowledgements
We thank B. Frydova for the help with maintaining animals in the breeding facility and T. Vendl for critical reading of the manuscript. We thank two anonymous reviewers for their constructive comments.
Funding
Open access publishing supported by the National Technical Library in Prague. This study was supported by the Ministry of Agriculture of the Czech Republic (Grant number MZe RO0418) and by the Ministry of the Interior of the Czech Republic (Grant number VH20182021038).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
All animal procedures were conducted in accordance with EU Directive 2010/63/EU for animal experiments, and ethical approval was obtained from the Institutional Animal Care and Use Committee of the Crop Research Institute. The experimental protocol was approved by the Ministry of the Environment of the Czech Republic (permit number MZP/2020/630/243).
Additional information
Communicated by Christian Imholt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Frankova, M., Radostna, T., Aulicky, R. et al. Less brodifacoum in baits results in greater accumulation in the liver of captive Rattus norvegicus in a no-choice trail. J Pest Sci (2024). https://doi.org/10.1007/s10340-023-01737-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10340-023-01737-y