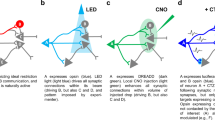
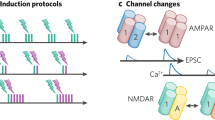
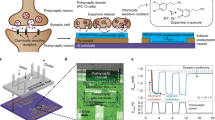
Abstract
Synapses are a key component of neural circuits, facilitating rapid and specific signalling between neurons. Synaptic engineering — the synthetic insertion of new synaptic connections into in vivo neural circuits — is an emerging approach for neural circuit interrogation. This approach is especially powerful for establishing causality in neural circuit structure–function relationships, for emulating synaptic plasticity and for exploring novel patterns of circuit connectivity. Contrary to other approaches for neural circuit manipulation, synaptic engineering targets specific connections between neurons and functions autonomously with no user-controlled external activation. Synaptic engineering has been successfully implemented in several systems and in different forms, including electrical synapses constructed from ectopically expressed connexin gap junction proteins, synthetic optical synapses composed of presynaptic photon-emitting luciferase coupled with postsynaptic light-gated channels, and artificial neuropeptide signalling pathways. This Perspective describes these different methods and how they have been applied, and examines how the field may advance.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hammond, C. & Esclapez, M. in Cellular and Molecular Neurophysiology 4th edn, Ch. 6 (Academic, 2015).
Vaughn, M. J. & Haas, J. S. On the diverse functions of electrical synapses. Front. Cell. Neurosci. 16, 910015 (2022).
van den Pol, A. N. Neuropeptide transmission in brain circuits. Neuron 76, 98–115 (2012).
Rabinowitch, I. & Schafer, W. R. Engineering new synaptic connections in the C. elegans connectome. Worm 4, e992668 (2015).
Roth, B. L. DREADDs for neuroscientists. Neuron 89, 683–694 (2016).
Chen, I. W., Papagiakoumou, E. & Emiliani, V. Towards circuit optogenetics. Curr. Opin. Neurobiol. 50, 179–189 (2018).
Shibata, A. C. E. et al. Photoactivatable CaMKII induces synaptic plasticity in single synapses. Nat. Commun. 12, 751 (2021).
Bergs, A. C. F. et al. All-optical closed-loop voltage clamp for precise control of muscles and neurons in live animals. Nat. Commun. 14, 1939 (2023).
Mahn, M., Prigge, M., Ron, S., Levy, R. & Yizhar, O. Biophysical constraints of optogenetic inhibition at presynaptic terminals. Nat. Neurosci. 19, 554–556 (2016).
Sanes, J. R. & Zipursky, S. L. Synaptic specificity, recognition molecules, and assembly of neural circuits. Cell 181, 536–556 (2020).
Faust, T. E., Gunner, G. & Schafer, D. P. Mechanisms governing activity-dependent synaptic pruning in the developing mammalian CNS. Nat. Rev. Neurosci. 22, 657–673 (2021).
Motz, C. T., Kabat, V., Saxena, T., Bellamkonda, R. V. & Zhu, C. Neuromechanobiology: an expanding field driven by the force of greater focus. Adv. Healthc. Mater. 10, 2100102 (2021).
Hassan, B. A. & Hiesinger, P. R. Beyond molecular codes: simple rules to wire complex brains. Cell 163, 285–291 (2015).
Sanes, J. R. & Yamagata, M. Many paths to synaptic specificity. Annu. Rev. Cell Dev. Biol. 25, 161–195 (2009).
Rabinowitch, I., Chatzigeorgiou, M., Zhao, B., Treinin, M. & Schafer, W. R. Rewiring neural circuits by the insertion of ectopic electrical synapses in transgenic C. elegans. Nat. Commun. 5, 4442 (2014).
Ransey, E. et al. Long-term precision editing of neural circuits in mammals using engineered gap junction hemichannels. Preprint at bioRxiv https://doi.org/10.1101/2021.08.24.457429 (2023).
Porta-de-la-Riva, M. et al. Neural engineering with photons as synaptic transmitters. Nat. Methods 20, 761–769 (2023).
Prakash, M. et al. Selective control of synaptically-connected circuit elements by all-optical synapses. Commun. Biol. 5, 33 (2022).
Hawk, J. D., Wisdom, E. M., Sengupta, T., Kashlan, Z. D. & Colón-Ramos, D. A. A genetically encoded tool for reconstituting synthetic modulatory neurotransmission and reconnect neural circuits in vivo. Nat. Commun. 12, 4795 (2021).
Ngo, H. B. et al. A chemogenetic tool that enables functional neural circuit analysis. Cell Rep. 32, 108139 (2020).
Kumar, N. M. & Gilula, N. B. The gap junction communication channel. Cell 84, 381–388 (1996).
Beyer, E. C. & Berthoud, V. M. Gap junction gene and protein families: connexins, innexins, and pannexins. Biochim. Biophys. Acta Biomembr. 1860, 5–8 (2018).
Skerrett, I. M. & Williams, J. B. A structural and functional comparison of gap junction channels composed of connexins and innexins. Dev. Neurobiol. 77, 522–547 (2017).
Chalasani, S. H. et al. Dissecting a circuit for olfactory behaviour in Caenorhabditis elegans. Nature 450, 63–70 (2007).
Rabinowitch, I., Chatzigeorgiou, M. & Schafer, W. R. A gap junction circuit enhances processing of coincident mechanosensory inputs. Curr. Biol. 23, 963–967 (2013).
Choi, U., Wang, H., Hu, M., Kim, S. & Sieburth, D. Presynaptic coupling by electrical synapses coordinates a rhythmic behavior by synchronizing the activities of a neuron pair. Proc. Natl Acad. Sci. USA 118, e2022599118 (2021).
Rabinowitch, I. et al. Neuropeptide-driven cross-modal plasticity following sensory loss in Caenorhabditis elegans. PLoS Biol. 14, e1002348 (2016).
Hawk, J. D. et al. Integration of plasticity mechanisms within a single sensory neuron of C. elegans actuates a memory. Neuron 97, 356–367.e4 (2018).
Choi, M. K., Liu, H., Wu, T., Yang, W. & Zhang, Y. NMDAR-mediated modulation of gap junction circuit regulates olfactory learning in C. elegans. Nat. Commun. 11, 3467 (2020).
Meng, L. & Yan, D. NLR-1/CASPR anchors F-actin to promote gap junction formation. Dev. Cell 55, 574–587.e3 (2020).
Pechuk, V. et al. Reprogramming the topology of the nociceptive circuit in C. elegans reshapes sexual behavior. Curr. Biol. 32, 4372–4385.e7 (2022).
Rabinowitch, I. et al. Circumventing neural damage in a C. elegans chemosensory circuit using genetically engineered synapses. Cell Syst. 12, 263–271.e4 (2021).
Almoril-Porras, A. et al. Specific configurations of electrical synapses filter sensory information to drive choices in behavior. Preprint at bioRxiv https://doi.org/10.1101/2023.08.01.551556 (2023).
Deisseroth, K. & Hegemann, P. The form and function of channelrhodopsin. Science 357, eaan5544 (2017).
Ernst, O. P. et al. Microbial and animal rhodopsins: structures, functions, and molecular mechanisms. Chem. Rev. 114, 126–163 (2014).
Govorunova, E. G., Sineshchekov, O. A., Li, H., Janz, R. & Spudich, J. L. Characterization of a highly efficient blue-shifted channelrhodopsin from the marine alga Platymonas subcordiformis. J. Biol. Chem. 288, 29911–29922 (2013).
Broser, M. et al. NeoR, a near-infrared absorbing rhodopsin. Nat. Commun. 11, 5682 (2020).
Klapoetke, N. C. et al. Independent optical excitation of distinct neural populations. Nat. Methods 11, 338–346 (2014).
Marshel, J. H. et al. Cortical layer-specific critical dynamics triggering perception. Science 365, eaaw5202 (2019).
Palombo, R. et al. Retinal chromophore charge delocalization and confinement explain the extreme photophysics of Neorhodopsin. Nat. Commun. 13, 6652 (2022).
Berglund, K., Fernandez, A. M., Gutekunst, C. A. N., Hochgeschwender, U. & Gross, R. E. Step-function luminopsins for bimodal prolonged neuromodulation. J. Neurosci. Res. 98, 422–436 (2020).
Wilson, T. & Woodland Hastings, J. Bioluminescence. Annu. Rev. Cell Dev. Biol. 14, 197–230 (1998).
Syed, A. J. & Anderson, J. C. Applications of bioluminescence in biotechnology and beyond. Chem. Soc. Rev. 50, 5668–5705 (2021).
Hall, M. P. et al. Engineered luciferase reporter from a deep sea shrimp utilizing a novel imidazopyrazinone substrate. ACS Chem. Biol. 7, 1848–1857 (2012).
Nagai, T. & Hattori, M. Tiny but bright. Nat. Rev. Chem. 6, 522–523 (2022).
England, C. G., Ehlerding, E. B. & Cai, W. NanoLuc: a small luciferase is brightening up the field of bioluminescence. Bioconjug. Chem. 27, 1175–1187 (2016).
Suzuki, K. et al. Five colour variants of bright luminescent protein for real-time multicolour bioimaging. Nat. Commun. 7, 13718 (2016).
Mitiouchkina, T. et al. Plants with genetically encoded autoluminescence. Nat. Biotechnol. 38, 944–946 (2020).
Bargmann, C. I. Beyond the connectome: how neuromodulators shape neural circuits. BioEssays 34, 458–465 (2012).
Findeisen, M., Rathmann, D. & Beck-Sickinger, A. G. RFamide peptides: structure, function, mechanisms and pharmaceutical potential. Pharmaceuticals 4, 1248–1280 (2011).
Carafoli, E. & Krebs, J. Why calcium? How calcium became the best communicator. J. Biol. Chem. 291, 20849–20857 (2016).
Connors, B. W. Synchrony and so much more: diverse roles for electrical synapses in neural circuits. Dev. Neurobiol. 77, 610–624 (2017).
Goodman, M. B., Hall, D. H., Avery, L. & Lockery, S. R. Active currents regulate sensitivity and dynamic range in C. elegans neurons. Neuron 20, 763–772 (1998).
Miller, A. C. & Pereda, A. E. The electrical synapse: molecular complexities at the gap and beyond. Dev. Neurobiol. 77, 562–574 (2017).
Phelan, P. et al. Molecular mechanism of rectification at identified electrical synapses in the Drosophila giant fiber system. Curr. Biol. 18, 1955–1960 (2008).
Ghosh, D. D. et al. Neural architecture of hunger-dependent multisensory decision making in C. elegans. Neuron 92, 1049–1062 (2016).
Morales-Curiel, L. F. et al. Volumetric imaging of fast cellular dynamics with deep learning enhanced bioluminescence microscopy. Commun. Biol. 5, 1330 (2022).
Cabré, G. et al. Rationally designed azobenzene photoswitches for efficient two-photon neuronal excitation. Nat. Commun. 10, 907 (2019).
Airan, R. D., Thompson, K. R., Fenno, L. E., Bernstein, H. & Deisseroth, K. Temporally precise in vivo control of intracellular signalling. Nature 458, 1025–1029 (2009).
Brittin, C. A., Cook, S. J., Hall, D. H., Emmons, S. W. & Cohen, N. A multi-scale brain map derived from whole-brain volumetric reconstructions. Nature 591, 105–110 (2021).
Moyle, M. W. et al. Structural and developmental principles of neuropil assembly in C. elegans. Nature 591, 99–104 (2021).
Ripoll-Sánchez, L. et al. The neuropeptidergic connectome of C. elegans. Neuron 111, 3570–3589.e5 (2023).
Schmitt, C., Schultheis, C., Husson, S. J., Liewald, J. F. & Gottschalk, A. Specific expression of channelrhodopsin-2 in single neurons of Caenorhabditis elegans. PLoS ONE 7, e43164 (2012).
Aoki, W. et al. Cellomics approach for high-throughput functional annotation of Caenorhabditis elegans neural network. Sci. Rep. 8, 10380 (2018).
Davis, L. et al. Precise optical control of gene expression in C. elegans using improved genetic code expansion and Cre recombinase. eLife 10, e67075 (2021).
Sáez, J. C., Retamal, M. A., Basilio, D., Bukauskas, F. F. & Bennett, M. V. L. Connexin-based gap junction hemichannels: gating mechanisms. Biochim. Biophys. Acta Biomembr. 1711, 215–224 (2005).
Emes, R. D. & Grant, S. G. N. Evolution of synapse complexity and diversity. Annu. Rev. Neurosci. 35, 111–131 (2012).
Hale, W. D., Südhof, T. C. & Huganir, R. L. Engineered adhesion molecules drive synapse organization. Proc. Natl Acad. Sci. USA 120, e2215905120 (2023).
Burlingham, S. R. et al. Induction of synapse formation by de novo neurotransmitter synthesis. Nat. Commun. 13, 3060 (2022).
Pirri, J. K., Rayes, D. & Alkema, M. J. A change in the ion selectivity of ligand-gated ion channels provides a mechanism to switch behavior. PLoS Biol. 13, e1002238 (2015).
Hashimshony, T., Feder, M., Levin, M., Hall, B. K. & Yanai, I. Spatiotemporal transcriptomics reveals the evolutionary history of the endoderm germ layer. Nature 519, 219–222 (2015).
Amin, J., Ananthan, J. & Voellmy, R. Key features of heat shock regulatory elements. Mol. Cell. Biol. 8, 3761–3769 (1988).
Kallunki, T., Barisic, M., Jäättelä, M. & Liu, B. How to choose the right inducible gene expression system for mammalian studies? Cells 8, 796 (2019).
Prozzillo, Y. et al. Targeted protein degradation tools: overview and future perspectives. Biology 9, 421 (2020).
Mishra, D. et al. An engineered protein-phosphorylation toggle network with implications for endogenous network discovery. Science 373, eaav0780 (2021).
Rabinowitch, I. What would a synthetic connectome look like? Phys. Life Rev. https://doi.org/10.1016/j.plrev.2019.06.005 (2019).
Palacios-Prado, N. & Bukauskas, F. F. Modulation of metabolic communication through gap junction channels by transjunctional voltage; synergistic and antagonistic effects of gating and ionophoresis. Biochim. Biophys. Acta 1818, 1884–1894 (2012).
Plahar, H. A. et al. BioParts — a biological parts search portal and updates to the ICE parts registry software platform. ACS Synth. Biol. 10, 2649–2660 (2021).
Rabinowitch, I. Synthetic biology in the brain: a vision of organic robots. In Proc. ALIFE 2019: the 2019 Conference on Artificial Life 654–655 (MIT Press, 2019).
Kukhtar, D. & Fussenegger, M. Synthetic biology in multicellular organisms: opportunities in nematodes. Biotechnol. Bioeng. 120, 2056–2071 (2023).
Rabinowitch, I. Inserting new synaptic connections into damaged neural circuits: towards synapse therapy? Neural Regen. Res. 17, 300–301 (2022).
Bassett, D. S. & Sporns, O. Network neuroscience. Nat. Neurosci. 20, 353–364 (2017).
Acknowledgements
I.R. received financial support from the Israel Science Foundation (grant 1465/20). Research in the D.A.C.-R. laboratory was supported by grants from NIH (R01NS076558), the National Science Foundation (NSF IOS 1353845) and DP1NS111778 and by an HHMI Scholar Award. M.K. acknowledges financial support from the ERC (MechanoSystems, 715243), HFSP (CDA00023/2018), MCIN/ AEI/10.13039/501100011033/ FEDER ‘A way to make Europe’, PID2021-123812OB-I00, ‘Severo Ochoa’ program for Centres of Excellence in R&D (CEX2019-000910-S), Fundació Privada Cellex, Fundació Mir-Puig and Generalitat de Catalunya through the CERCA and Research program.
Author information
Authors and Affiliations
Contributions
The authors all contributed to all aspects of the article preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Neuroscience thanks J. Dittman and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rabinowitch, I., Colón-Ramos, D.A. & Krieg, M. Understanding neural circuit function through synaptic engineering. Nat. Rev. Neurosci. 25, 131–139 (2024). https://doi.org/10.1038/s41583-023-00777-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41583-023-00777-8