Abstract
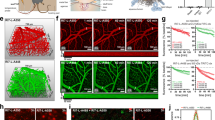
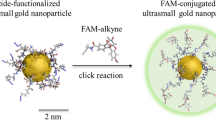
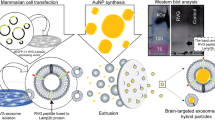
Nanoparticles (NPs) represent an important advance for delivering diagnostic and therapeutic agents across the blood–brain barrier. However, NP clearance is critical for safety and therapeutic applicability. Here we report on a study of the clearance of model organic and inorganic NPs from the brain. We find that microglial extracellular vesicles (EVs) play a crucial role in the clearance of inorganic and organic NPs from the brain. Inorganic NPs, unlike organic NPs, perturb the biogenesis of microglial EVs through the inhibition of ERK1/2 signalling. This increases the accumulation of inorganic NPs in microglia, hindering their elimination via the paravascular route. We also demonstrate that stimulating the release of microglial EVs by an ERK1/2 activator increased the paravascular glymphatic pathway-mediated brain clearance of inorganic NPs. These findings highlight the modulatory role of microglial EVs on the distinct patterns of the clearance of organic and inorganic NPs from the brain and provide a strategy for modulating the intracerebral fate of NPs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the Article and the Supplementary Information. All RNA sequence raw data were deposited to the NCBI Sequence Read Archive (https://www.ncbi.nlm.nih.gov/sra) with the identifier BioProject ID: PRJNA1003397. Proteomics raw data have been deposited to the PRIDE archive (https://www.ebi.ac.uk/pride/archive) with the identifier PXD044395. Source data are provided with this paper.
References
Zheng, M., Tao, W., Zou, Y., Farokhzad, O. C. & Shi, B. Nanotechnology-based strategies for siRNA brain delivery for disease therapy. Trends Biotechnol. 36, 562–575 (2018).
Poon, W., Kingston, B. R., Ouyang, B., Ngo, W. & Chan, W. C. W. A framework for designing delivery systems. Nat. Nanotechnol. 15, 819–829 (2020).
Mitchell, M. J. et al. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 20, 101–124 (2021).
Chertok, B. et al. Iron oxide nanoparticles as a drug delivery vehicle for MRI monitored magnetic targeting of brain tumors. Biomaterials 29, 487–496 (2008).
Huang, H., Feng, W., Chen, Y. & Shi, J. L. Inorganic nanoparticles in clinical trials and translations. Nano Today 35, 100972 (2020).
Zhao, P., Le, Z., Liu, L. & Chen, Y. Therapeutic delivery to the brain via the lymphatic vasculature. Nano Lett. 20, 5415–5420 (2020).
Ma, F. et al. Neurotransmitter-derived lipidoids (NT-lipidoids) for enhanced brain delivery through intravenous injection. Sci. Adv. 6, eabb4429 (2020).
Terstappen, G. C., Meyer, A. H., Bell, R. D. & Zhang, W. Strategies for delivering therapeutics across the blood–brain barrier. Nat. Rev. Drug Discov. 20, 362–383 (2021).
Cheon, J., Chan, W. & Zuhorn, I. The future of nanotechnology: cross-disciplined progress to improve health and medicine. Acc. Chem. Res. 52, 2405 (2019).
Stater, E. P., Sonay, A. Y., Hart, C. & Grimm, J. The ancillary effects of nanoparticles and their implications for nanomedicine. Nat. Nanotechnol. 16, 1180–1194 (2021).
Hawkins, S. J. et al. Nanoparticle-induced neuronal toxicity across placental barriers is mediated by autophagy and dependent on astrocytes. Nat. Nanotechnol. 13, 427–433 (2018).
Khan, A. M. et al. Silver nanoparticle-induced expression of proteins related to oxidative stress and neurodegeneration in an in vitro human blood–brain barrier model. Nanotoxicology 13, 221–239 (2019).
Buchman, J. T., Hudson-Smith, N. V., Landy, K. M. & Haynes, C. L. Understanding nanoparticle toxicity mechanisms to inform redesign strategies to reduce environmental impact. Acc. Chem. Res. 52, 1632–1642 (2019).
Li, L. et al. Silver nanoparticles induce protective autophagy via Ca2+/CaMKKβ/AMPK/mTOR pathway in SH-SY5Y cells and rat brains. Nanotoxicology 13, 369–391 (2019).
Li, Y. & Ju, D. The role of autophagy in nanoparticles-induced toxicity and its related cellular and molecular mechanisms. Adv. Exp. Med. Biol. 1048, 71–84 (2018).
Onoda, A., Kawasaki, T., Tsukiyama, K., Takeda, K. & Umezawa, M. Carbon nanoparticles induce endoplasmic reticulum stress around blood vessels with accumulation of misfolded proteins in the developing brain of offspring. Sci. Rep. 10, 10028 (2020).
Maher, B. A. et al. Magnetite pollution nanoparticles in the human brain. Proc. Natl Acad. Sci. USA 113, 10797–10801 (2016).
Khlebtsov, N. & Dykman, L. Biodistribution and toxicity of engineered gold nanoparticles: a review of in vitro and in vivo studies. Chem. Soc. Rev. 40, 1647–1671 (2011).
Skotland, T., Iversen, T. G., Llorente, A. & Sandvig, K. Biodistribution, pharmacokinetics and excretion studies of intravenously injected nanoparticles and extracellular vesicles: possibilities and challenges. Adv. Drug Deliv. Rev. 186, 114326 (2022).
Wei, Y. C., Quan, L., Zhou, C. & Zhan, Q. Q. Factors relating to the biodistribution & clearance of nanoparticles & their effects on in vivo application. Nanomedicine 13, 1495–1512 (2018).
Yang, G. et al. A hypoxia-responsive albumin-based nanosystem for deep tumor penetration and excellent therapeutic efficacy. Adv. Mater. 31, e1901513 (2019).
He, C. F. et al. Advances in biodegradable nanomaterials for photothermal therapy of cancer. Cancer Biol. Med. 13, 299–312 (2016).
Tosi, G. et al. Insight on the fate of CNS-targeted nanoparticles. Part II: intercellular neuronal cell-to-cell transport. J. Control. Release 177, 96–107 (2014).
Borst, K., Dumas, A. A. & Prinz, M. Microglia: immune and non-immune functions. Immunity 54, 2194–2208 (2021).
Bourquin, J. et al. Biodistribution, clearance, and long-term fate of clinically relevant nanomaterials. Adv. Mater. 30, e1704307 (2018).
Gu, X. et al. Clearance of two organic nanoparticles from the brain via the paravascular pathway. J. Control. Release 322, 31–41 (2020).
Tarasoff-Conway, J. M. et al. Clearance systems in the brain—implications for Alzheimer disease. Nat. Rev. Neurol. 11, 457–470 (2015).
Iliff, J. J. et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 4, 147ra111 (2012).
Meng, X. et al. The biological fate of the polymer nanocarrier material monomethoxy poly(ethylene glycol)-block-poly(d,l-lactic acid) in rat. Acta Pharm. Sin. B 11, 1003–1009 (2021).
Antsiferova, A. A., Kopaeva, M. Y., Kochkin, V. N. & Kashkarov, P. K. Kinetics of silver accumulation in tissues of laboratory mice after long-term oral administration of silver nanoparticles. Nanomaterials (Basel) 11, 3204 (2021).
El-Drieny, E. et al. Histological and immunohistochemical study of the effect of gold nanoparticles on the brain of adult male albino rat. J. Microsc Ultrastruct. 3, 181–190 (2015).
Ferreira, G. K. et al. Effect of acute and long-term administration of gold nanoparticles on biochemical parameters in rat brain. Mater. Sci. Eng. C 79, 748–755 (2017).
Arezki, Y. et al. Surface charge influences protein corona, cell uptake and biological effects of carbon dots. Nanoscale 14, 14695–14710 (2022).
Georgieva, J. V. et al. Surface characteristics of nanoparticles determine their intracellular fate in and processing by human blood–brain barrier endothelial cells in vitro. Mol. Ther. 19, 318–325 (2011).
Weng, J. W. et al. Mediating bio-fate of polymeric cholecalciferol nanoparticles through rational size control. Biomater. Adv. 140, 213074 (2022).
Parhiz, H. et al. Unintended effects of drug carriers: big issues of small particles. Adv. Drug Deliv. Rev. 130, 90–112 (2018).
Datta, A. et al. Manumycin A suppresses exosome biogenesis and secretion via targeted inhibition of Ras/Raf/ERK1/2 signaling and hnRNP H1 in castration-resistant prostate cancer cells. Cancer Lett. 408, 73–81 (2017).
Ren, J. & Guo, W. ERK1/2 regulate exocytosis through direct phosphorylation of the exocyst component Exo70. Dev. Cell 22, 967–978 (2012).
Hsu, S. C., TerBush, D., Abraham, M. & Guo, W. The exocyst complex in polarized exocytosis. Int. Rev. Cytol. 233, 243–265 (2004).
Aikawa, Y. & Martin, T. F. ARF6 regulates a plasma membrane pool of phosphatidylinositol(4,5)bisphosphate required for regulated exocytosis. J. Cell Biol. 162, 647–659 (2003).
Yeh, Y. C., Lin, Y. P., Kramer, H. & Parekh, A. B. Single-nucleotide polymorphisms in Orai1 associated with atopic dermatitis inhibit protein turnover, decrease calcium entry and disrupt calcium-dependent gene expression. Hum. Mol. Genet. 29, 1808–1823 (2020).
McAndrews, K. M., LeBleu, V. S. & Kalluri, R. SIRT1 regulates lysosome function and exosome secretion. Dev. Cell 49, 302–303 (2019).
Polanco, J. C., Hand, G. R., Briner, A., Li, C. Z. & Gotz, J. Exosomes induce endolysosomal permeabilization as a gateway by which exosomal tau seeds escape into the cytosol. Acta Neuropathol. 141, 235–256 (2021).
Trajkovic, K. et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319, 1244–1247 (2008).
Iguchi, Y. et al. Exosome secretion is a key pathway for clearance of pathological TDP-43. Brain 139, 3187–3201 (2016).
Isaac, R., Reis, F. C. G., Ying, W. & Olefsky, J. M. Exosomes as mediators of intercellular crosstalk in metabolism. Cell Metab. 33, 1744–1762 (2021).
Song, Q. et al. Lipoprotein-based nanoparticles rescue the memory loss of mice with Alzheimer’s disease by accelerating the clearance of amyloid-β. ACS Nano 8, 2345–2359 (2014).
Yao, L. et al. Nanoformulated α-mangostin ameliorates Alzheimer’s disease neuropathology by elevating LDLR expression and accelerating amyloid-β clearance. J. Control. Release 226, 1–14 (2016).
Thery, C., Amigorena, S., Raposo, G. & Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. https://doi.org/10.1002/0471143030.cb0322s30 (2006).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81973272, 92068111, 82073836, 82171358), the National Key Research and Development Program of China (2022YFC2502800), the Canada Research Chairs Program (950-232468) and grants from the Shanghai Science and Technology Committee (21XD1422200, 23S41900100) and the Innovation Program of Shanghai Municipal Education Commission (2023ZKZD21). We thank Y. Wu, G. Li and Y. Huang from the Core Facility of Basic Medical Sciences, Shanghai Jiao Tong University School of Medicine. We are also grateful to J. Chen from the Instrumental Analysis Center of Shanghai Jiao Tong University for providing instrumental assistance.
Author information
Authors and Affiliations
Contributions
J.G. and X. Gao are responsible for all phases of the research. J.G. designed and executed the experiments, analysed the data and wrote the original manuscript. X. Gao conceptualized and supervised the project and contributed to the experimental planning, data analysis and manuscript revision. H.C. and G.Z. conceptualized and supervised the project and contributed to the manuscript revision. Q.S. and X. Gu helped in designing and executing the in vivo two-photon laser-scanning microscopy experiments. G.J., J.H. and A.W. helped with the preparation of organic nanoparticles. Y.T. helped with the extraction of exosomes. R.Y. and Y.H. helped with in vitro BV-2 cell studies. All authors reviewed and approved the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Elizabeth Nance and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–29, Discussion and Tables 1–6.
Supplementary Video 1
DiD-rHDL uptake (0–1 h). BV-2 cells were exposed to DMEM with 1% DiD-rHDL (25 μg/ml) for 1 h. Cells were stained with Hoechst 33342 (blue).
Supplementary Video 2
DiD-PEG-PLA NPs uptake (0–1 h). BV-2 cells were exposed to DMEM with 1% DiD-PEG-PLA (50 μg/ml) for 1 h. Cells were stained with Hoechst 33342 (blue).
Supplementary Video 3
QD uptake (0–1 h). BV-2 cells were exposed to DMEM with QD (30 μg/ml) for 1 h. Cells were stained with Hoechst 33342 (blue).
Supplementary Video 4
PQD uptake (0–1 h). BV-2 cells were exposed to DMEM with PQD (30 μg/ml) for 1 h. Cells were stained with Hoechst 33342 (blue).
Supplementary Video 5
DiD-rHDL exocytosis (1–4 h). BV-2 cells were exposed to DMEM with 1% DiD-rHDL (25 μg/ml) (red) for 1 h. Then cells were gently washed twice with the fresh medium and incubated in the fresh culture medium for exocytosis for 3 h. BV-2 cells were stained with Lyso-Tracker (green) and Hoechst 33342 (blue).
Supplementary Video 6
DiD-PEG-PLA NPs exocytosis (1–4 h). BV-2 cells were exposed to DMEM with 1% DiD-PEG-PLA (50 μg/ml) (red) for 1 h. Then cells were gently washed twice with the fresh medium and incubated in the fresh culture medium for exocytosis for 3 h. BV-2 cells were stained with Lyso-Tracker (green) and Hoechst 33342 (blue).
Supplementary Video 7
QD exocytosis (1–4 h). BV-2 cells were exposed to DMEM with QD (30 μg/ml) (red) for 1 h. Then cells were gently washed twice with the fresh medium and incubated in the fresh culture medium for exocytosis for 3 h. BV-2 cells were stained with Lyso-Tracker (green) and Hoechst 33342 (blue).
Supplementary Video 8
PQD exocytosis (1–4 h). BV-2 cells were exposed to DMEM with PQD (30 μg/ml) (red) for 1 h. Then cells were gently washed twice with the fresh medium and incubated in the fresh culture medium for exocytosis for 3 h. BV-2 cells were stained with Lyso-Tracker (green) and Hoechst 33342 (blue).
Supplementary Data 1
Statistical source data of Supplementary Figures.
Supplementary Data 2
Unprocessed western blots for Supplementary Figures.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 3
Unprocessed western blots.
Source Data Fig. 4
Unprocessed western blots.
Source Data Fig. 5
Unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, J., Song, Q., Gu, X. et al. Intracerebral fate of organic and inorganic nanoparticles is dependent on microglial extracellular vesicle function. Nat. Nanotechnol. 19, 376–386 (2024). https://doi.org/10.1038/s41565-023-01551-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-023-01551-8
This article is cited by
-
Intracerebral fate of engineered nanoparticles
Nature Nanotechnology (2024)
-
Recent advances in molecular and nanoparticle probes for fluorescent bioanalysis
Nano Research (2024)