Abstract
Sleep is considered essential for the brain and body. A predominant concept is that sleep is regulated by circadian rhythmicity and sleep homeostasis, processes that were posited to be functionally and mechanistically separate. Here we review and re-evaluate this concept and its assumptions using findings from recent human and rodent studies. Alterations in genes that are central to circadian rhythmicity affect not only sleep timing but also putative markers of sleep homeostasis such as electroencephalogram slow-wave activity (SWA). Perturbations of sleep change the rhythmicity in the expression of core clock genes in tissues outside the central clock. The dynamics of recovery from sleep loss vary across sleep variables: SWA and immediate early genes show an early response, but the recovery of non-rapid eye movement and rapid eye movement sleep follows slower time courses. Changes in the expression of many genes in response to sleep perturbations outlast the effects on SWA and time spent asleep. These findings are difficult to reconcile with the notion that circadian- and sleep–wake-driven processes are mutually independent and that the dynamics of sleep homeostasis are reflected in a single variable. Further understanding of how both sleep and circadian rhythmicity contribute to the homeostasis of essential physiological variables may benefit from the assessment of multiple sleep and molecular variables over longer time scales.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
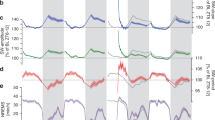
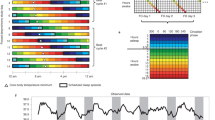
The example graphs depicted in Figs. 1 and 2 are based on the publicly accessible data resource https://bxd.vital-it.ch/, published first in ref. 39 and then as a digital research object describing the analyses pipe-line and links to data and analyses scripts98. Simulations of the sleep–wake-driven process in the mouse (Fig. 4b,d) were performed using parameters and methods described in ref. 99 and illustrated in Fig. 4a, on data from ref. 100.
References
Girardeau, G. & Lopes-Dos-Santos, V. Brain neural patterns and the memory function of sleep. Science 374, 560–564 (2021).
Frank, M. G. & Heller, H. C. The function(s) of sleep. Handb. Exp. Pharmacol. 253, 3–34 (2019). A very accessible and comprehensive review of current thinking and evidence concerning the functions of sleep.
Hertenstein, E., Benz, F., Schneider, C. & Baglioni, C. Insomnia—a risk factor for mental disorders. J. Sleep. Res. 22, e13930 (2023).
Wu, T. T. et al. Insomnia and multiple health outcomes: umbrella review of meta-analyses of prospective cohort studies. Public. Health 215, 66–74 (2023).
Koronowski, K. B. & Sassone-Corsi, P. Communicating clocks shape circadian homeostasis. Science 371, eabd0951 (2021). This review highlights the concept of ‘circadian homeostasis’ by putting the network of circadian circuitries present in various tissues into a homeostatic context.
Hastings, M. H., Maywood, E. S. & Brancaccio, M. The mammalian circadian timing system and the suprachiasmatic nucleus as its pacemaker. Biology 8, 13 (2019).
Tu, B. P. & McKnight, S. L. Metabolic cycles as an underlying basis of biological oscillations. Nat. Rev. Mol. Cell Biol. 7, 696–701 (2006).
Daan, S., Beersma, D. G. & Borbely, A. A. Timing of human sleep: recovery process gated by a circadian pacemaker. Am. J. Physiol. 246, R161–R183 (1984). This paper provides a quantitative exploration of how the interaction between sleep homeostasis and circadian rhythmicity explains a broad range of sleep phenomena.
Borbely, A. A. A two process model of sleep regulation. Hum. Neurobiol. 1, 195–204 (1982).
Edgar, D. M., Dement, W. C. & Fuller, C. A. Effect of SCN lesions on sleep in squirrel monkeys: evidence for opponent processes in sleep-wake regulation. J. Neurosci. 13, 1065–1079 (1993).
Shafer, O. T. & Keene, A. C. The regulation of Drosophila sleep. Curr. Biol. 31, R38–R49 (2021).
Chiu, C. N. & Prober, D. A. Regulation of zebrafish sleep and arousal states: current and prospective approaches. Front. Neural Circuits 7, 58 (2013).
Krueger, J. M., Nguyen, J. T., Dykstra-Aiello, C. J. & Taishi, P. Local sleep. Sleep. Med. Rev. 43, 14–21 (2019).
Noya, S. B. et al. The forebrain synaptic transcriptome is organized by clocks but its proteome is driven by sleep. Science 366, eaav2642 (2019).
Delorme, J. et al. Hippocampal neurons’ cytosolic and membrane-bound ribosomal transcript profiles are differentially regulated by learning and subsequent sleep. Proc. Natl Acad. Sci. USA 118, e2108534118 (2021).
Bellesi, M., de Vivo, L., Tononi, G. & Cirelli, C. Effects of sleep and wake on astrocytes: clues from molecular and ultrastructural studies. BMC Biol. 13, 66 (2015).
Zepelin, H. S. & Tobler, I. in Principles and Practise of Sleep Medicine, 4th edition (ed. Roth Kryger, D.) Ch. 8, 91–100 (Elsevier Saunders, 2005).
Barbato, G., Barker, C., Bender, C. & Wehr, T. A. Spontaneous sleep interruptions during extended nights. Relationships with NREM and REM sleep phases and effects on REM sleep regulation. Clin. Neurophysiol. 113, 892–900 (2002).
Simor, P., van der Wijk, G., Nobili, L. & Peigneux, P. The microstructure of REM sleep: why phasic and tonic. Sleep. Med. Rev. 52, 101305 (2020).
Stephan, A. M., Lecci, S., Cataldi, J. & Siclari, F. Conscious experiences and high-density EEG patterns predicting subjective sleep depth. Curr. Biol. 31, 5487–5500.e3 (2021).
Osorio-Forero, A. et al. Noradrenergic circuit control of non-REM sleep substates. Curr. Biol. 31, 5009–5023.e7 (2021).
Lecci, S. et al. Electroencephalographic changes associated with subjective under- and overestimation of sleep duration. Sleep 43, zsaa094 (2020).
Cajochen, C., Wyatt, J. K., Czeisler, C. A. & Dijk, D. J. Separation of circadian and wake duration-dependent modulation of EEG activation during wakefulness. Neuroscience 114, 1047–1060 (2002).
Davis, C. J., Clinton, J. M., Jewett, K. A., Zielinski, M. R. & Krueger, J. M. Delta wave power: an independent sleep phenotype or epiphenomenon? J. Clin. Sleep. Med. 7, S16–S18 (2011).
Wang, D. et al. Slow wave sleep in patients with respiratory failure. Sleep. Med. 12, 378–383 (2011).
Aeschbach, D., Dijk, D. J., Trachsel, L., Brunner, D. P. & Borbely, A. A. Dynamics of slow-wave activity and spindle frequency activity in the human sleep EEG: effect of midazolam and zopiclone. Neuropsychopharmacology 11, 237–244 (1994).
Franken, P., Dijk, D. J., Tobler, I. & Borbely, A. A. Sleep deprivation in rats: effects on EEG power spectra, vigilance states, and cortical temperature. Am. J. Physiol. 261, R198–R208 (1991).
Achermann, P., Dijk, D. J., Brunner, D. P. & Borbely, A. A. A model of human sleep homeostasis based on EEG slow-wave activity: quantitative comparison of data and simulations. Brain Res. Bull. 31, 97–113 (1993).
Thomas, C. W., Guillaumin, M. C., McKillop, L. E., Achermann, P. & Vyazovskiy, V. V. Global sleep homeostasis reflects temporally and spatially integrated local cortical neuronal activity. eLife 9, e54148 (2020).
Franken, P., Tobler, I. & Borbely, A. A. Sleep homeostasis in the rat: simulation of the time course of EEG slow-wave activity. Neurosci. Lett. 130, 141–144 (1991). This paper provides a quantitative account of how the distribution of sleep and wakefulness drives the time course of slow wave activity in the rat.
Franken, P., Chollet, D. & Tafti, M. The homeostatic regulation of sleep need is under genetic control. J. Neurosci. 21, 2610–2621 (2001).
Rusterholz, T., Durr, R. & Achermann, P. Inter-individual differences in the dynamics of sleep homeostasis. Sleep 33, 491–498 (2010).
Huber, R., Tononi, G. & Cirelli, C. Exploratory behavior, cortical BDNF expression, and sleep homeostasis. Sleep 30, 129–139 (2007).
Vassalli, A. & Franken, P. Hypocretin (orexin) is critical in sustaining theta/gamma-rich waking behaviors that drive sleep need. Proc. Natl Acad. Sci. USA 114, E5464–E5473 (2017).
Dijk, D. J., Brunner, D. P. & Borbely, A. A. Time course of EEG power density during long sleep in humans. Am. J. Physiol. 258, R650–R661 (1990).
Hubbard, J. et al. Rapid fast-delta decay following prolonged wakefulness marks a phase of wake-inertia in NREM sleep. Nat. Commun. 11, 3130 (2020).
Rosenthal, L., Merlotti, L., Roehrs, T. A. & Roth, T. Enforced 24-hour recovery following sleep deprivation. Sleep 14, 448–453 (1991).
Franken, P., Malafosse, A. & Tafti, M. Genetic determinants of sleep regulation in inbred mice. Sleep 22, 155–169 (1999).
Diessler, S. et al. A systems genetics resource and analysis of sleep regulation in the mouse. PLoS Biol. 16, e2005750 (2018).
McCarthy, A. et al. REM sleep homeostasis in the absence of REM sleep: effects of antidepressants. Neuropharmacology 108, 415–425 (2016).
Amici, R. et al. Cold exposure and sleep in the rat: REM sleep homeostasis and body size. Sleep 31, 708–715 (2008).
Skorucak, J., Arbon, E. L., Dijk, D. J. & Achermann, P. Response to chronic sleep restriction, extension, and subsequent total sleep deprivation in humans: adaptation or preserved sleep homeostasis? Sleep https://doi.org/10.1093/sleep/zsy078 (2018).
Endo, T. et al. Selective REM sleep deprivation in humans: effects on sleep and sleep EEG. Am. J. Physiol. 274, R1186–R1194 (1998).
Trachsel, L., Edgar, D. M., Seidel, W. F., Heller, H. C. & Dement, W. C. Sleep homeostasis in suprachiasmatic nuclei-lesioned rats: effects of sleep deprivation and triazolam administration. Brain Res. 589, 253–261 (1992).
Borbely, A. A., Baumann, F., Brandeis, D., Strauch, I. & Lehmann, D. Sleep deprivation: effect on sleep stages and EEG power density in man. Electroencephalogr. Clin. Neurophysiol. 51, 483–495 (1981).
Dijk, D. J. & Czeisler, C. A. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J. Neurosci. 15, 3526–3538 (1995). This paper provides analyses of a forced desynchrony protocol that demonstrates that many aspects of sleep are influenced by both sleep-dependent and circadian processes, but to a different extent, and that the processes interact.
Franken, P. Long-term vs. short-term processes regulating REM sleep. J. Sleep. Res. 11, 17–28 (2002).
Park, S. H. & Weber, F. Neural and homeostatic regulation of REM sleep. Front. Psychol. 11, 1662 (2020).
Ocampo-Garces, A., Bassi, A., Brunetti, E., Estrada, J. & Vivaldi, E. A. REM sleep-dependent short-term and long-term hourglass processes in the ultradian organization and recovery of REM sleep in the rat. Sleep 43, zsaa023 (2020).
Park, S. H. et al. A probabilistic model for the ultradian timing of REM sleep in mice. PLoS Comput. Biol. 17, e1009316 (2021).
Barbato, G. & Wehr, T. A. Homeostatic regulation of REM sleep in humans during extended sleep. Sleep 21, 267–276 (1998).
Dijk, D. J., Beersma, D. G. & Daan, S. EEG power density during nap sleep: reflection of an hourglass measuring the duration of prior wakefulness. J. Biol. Rhythm. 2, 207–219 (1987).
Werth, E., Dijk, D. J., Achermann, P. & Borbely, A. A. Dynamics of the sleep EEG after an early evening nap: experimental data and simulations. Am. J. Physiol. 271, R501–R510 (1996).
Vyazovskiy, V. V., Achermann, P. & Tobler, I. Sleep homeostasis in the rat in the light and dark period. Brain Res. Bull. 74, 37–44 (2007).
Huber, R., Deboer, T. & Tobler, I. Effects of sleep deprivation on sleep and sleep EEG in three mouse strains: empirical data and simulations. Brain Res. 857, 8–19 (2000).
Achermann, P. & Borbely, A. A. Low-frequency (<1 Hz) oscillations in the human sleep electroencephalogram. Neuroscience 81, 213–222 (1997).
Van Dongen, H. P., Maislin, G., Mullington, J. M. & Dinges, D. F. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep 26, 117–126 (2003).
Mohawk, J. A., Green, C. B. & Takahashi, J. S. Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci. 35, 445–462 (2012).
Hastings, M. H., Maywood, E. S. & Brancaccio, M. Generation of circadian rhythms in the suprachiasmatic nucleus. Nat. Rev. Neurosci. 19, 453–469 (2018).
Lazar, A. S., Lazar, Z. I. & Dijk, D. J. Circadian regulation of slow waves in human sleep: topographical aspects. Neuroimage 116, 123–134 (2015).
Yasenkov, R. & Deboer, T. Interrelations and circadian changes of electroencephalogram frequencies under baseline conditions and constant sleep pressure in the rat. Neuroscience 180, 212–221 (2011).
Mistlberger, R. E. Circadian regulation of sleep in mammals: role of the suprachiasmatic nucleus. Brain Res. Brain Res. Rev. 49, 429–454 (2005).
Maywood, E. S., Chesham, J. E., Winsky-Sommerer, R. & Hastings, M. H. Restoring the molecular clockwork within the suprachiasmatic hypothalamus of an otherwise clockless mouse enables circadian phasing and stabilization of sleep-wake cycles and reverses memory deficits. J. Neurosci. 41, 8562–8576 (2021). This paper demonstrates that restoring rhythmicity in the SCN through local genetic complementation restores rhythmicity in sleep and wakefulness, REM sleep and slow wave activity, indicating the predominant role of the SCN in driving rhythms in sleep–wake behaviour, even in the absence of peripheral clocks.
Wisor, J. P. et al. A role for cryptochromes in sleep regulation. BMC Neurosci. 3, 20 (2002).
Laposky, A. et al. Deletion of the mammalian circadian clock gene BMAL1/Mop3 alters baseline sleep architecture and the response to sleep deprivation. Sleep 28, 395–409 (2005).
Yu, X. et al. Circadian factor BMAL1 in histaminergic neurons regulates sleep architecture. Curr. Biol. 24, 2838–2844 (2014).
Ehlen, J. C. et al. Bmal1 function in skeletal muscle regulates sleep. eLife 6, e26557 (2017).
Wang, W. et al. Desynchronizing the sleep–wake cycle from circadian timing to assess their separate contributions to physiology and behaviour and to estimate intrinsic circadian period. Nat. Protoc. 18, 579–603 (2023).
Cambras, T. et al. Circadian desynchronization of core body temperature and sleep stages in the rat. Proc. Natl Acad. Sci. USA 104, 7634–7639 (2007).
Maywood, E. S., Chesham, J. E., Winsky-Sommerer, R., Smyllie, N. J. & Hastings, M. H. Circadian chimeric mice reveal an interplay between the suprachiasmatic nucleus and local brain clocks in the control of sleep and memory. Front. Neurosci. 15, 639281 (2021). This paper provides an analysis of sleep–wake characteristics and response to sleep loss and sleep-dependent memory in mice in which the period of peripheral clocks is different from the SCN and demonstrates a contribution of peripheral clocks despite the dominant role of the SCN.
Dijk, D. J., Duffy, J. F., Riel, E., Shanahan, T. L. & Czeisler, C. A. Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. J. Physiol. 516, 611–627 (1999).
Akerstedt, T. & Gillberg, M. The circadian variation of experimentally displaced sleep. Sleep 4, 159–169 (1981).
Czeisler, C. A., Weitzman, E., Moore-Ede, M. C., Zimmerman, J. C. & Knauer, R. S. Human sleep: its duration and organization depend on its circadian phase. Science 210, 1264–1267 (1980).
Dijk, D. J., Shanahan, T. L., Duffy, J. F., Ronda, J. M. & Czeisler, C. A. Variation of electroencephalographic activity during non-rapid eye movement and rapid eye movement sleep with phase of circadian melatonin rhythm in humans. J. Physiol. 505, 851–858 (1997).
Munch, M., Silva, E. J., Ronda, J. M., Czeisler, C. A. & Duffy, J. F. EEG sleep spectra in older adults across all circadian phases during NREM sleep. Sleep 33, 389–401 (2010).
Vyazovskiy, V. V., Cirelli, C., Pfister-Genskow, M., Faraguna, U. & Tononi, G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat. Neurosci. 11, 200–208 (2008).
Curie, T. et al. Homeostatic and circadian contribution to EEG and molecular state variables of sleep regulation. Sleep 36, 311–323 (2013).
Chang, A. M. et al. Circadian gene variants influence sleep and the sleep electroencephalogram in humans. Chronobiol. Int. 33, 561–573 (2016).
Duffy, J. F. & Dijk, D. J. Getting through to circadian oscillators: why use constant routines. J. Biol. Rhythm. 17, 4–13 (2002).
Sela, Y., Hoekstra, M. M. & Franken, P. Sub-minute prediction of brain temperature based on sleep-wake state in the mouse. eLife 10, e62073 (2021).
Franken, P., Tobler, I. & Borbely, A. A. Sleep and waking have a major effect on the 24-hr rhythm of cortical temperature in the rat. J. Biol. Rhythm. 7, 341–352 (1992).
Masubuchi, S. et al. Clock genes outside the suprachiasmatic nucleus involved in manifestation of locomotor activity rhythm in rats. Eur. J. Neurosci. 12, 4206–4214 (2000).
Abe, H., Honma, S., Namihira, M., Masubuchi, S. & Honma, K. Behavioural rhythm splitting in the CS mouse is related to clock gene expression outside the suprachiasmatic nucleus. Eur. J. Neurosci. 14, 1121–1128 (2001).
Wakamatsu, H. et al. Restricted-feeding-induced anticipatory activity rhythm is associated with a phase-shift of the expression of mPer1 and mPer2 mRNA in the cerebral cortex and hippocampus but not in the suprachiasmatic nucleus of mice. Eur. J. Neurosci. 13, 1190–1196 (2001).
Dudley, C. A. et al. Altered patterns of sleep and behavioral adaptability in NPAS2-deficient mice. Science 301, 379–383 (2003).
Le Minh, N., Damiola, F., Tronche, F., Schutz, G. & Schibler, U. Glucocorticoid hormones inhibit food-induced phase-shifting of peripheral circadian oscillators. EMBO J. 20, 7128–7136 (2001).
Hoekstra, M. M., Jan, M., Katsioudi, G., Emmenegger, Y. & Franken, P. The sleep-wake distribution contributes to the peripheral rhythms in PERIOD-2. eLife 10, e69773 (2021).
Hor, C. N. et al. Sleep-wake-driven and circadian contributions to daily rhythms in gene expression and chromatin accessibility in the murine cortex. Proc. Natl Acad. Sci. USA 116, 25773–25783 (2019). This paper describes multiday time course analyses of gene expression and chromatin accessibility in the mouse cortex, quantifying the sleep–wake-driven and circadian contributions to gene expression dynamics. The study discovered a surprisingly long effect of sleep loss on clock gene rhythms.
Archer, S. N. et al. Mistimed sleep disrupts circadian regulation of the human transcriptome. Proc. Natl Acad. Sci. USA 111, E682–E691 (2014). This study used a forced desynchrony protocol and showed that the appropriate circadian timing of sleep makes an important contribution to the rhythmic changes in gene expression in the human blood transcriptome.
Schibler, U. et al. Clock-talk: interactions between central and peripheral circadian oscillators in mammals. Cold Spring Harb. Symp. Quant. Biol. 80, 223–232 (2015). This article reviews the signalling pathways by which the SCN synchronizes circadian rhythms in peripheral organs.
Curie, T., Maret, S., Emmenegger, Y. & Franken, P. In vivo imaging of the central and peripheral effects of sleep deprivation and suprachiasmatic nuclei lesion on PERIOD-2 protein in mice. Sleep 38, 1381–1394 (2015).
Deboer, T., Vansteensel, M. J., Detari, L. & Meijer, J. H. Sleep states alter activity of suprachiasmatic nucleus neurons. Nat. Neurosci. 6, 1086–1090 (2003).
Deboer, T., Detari, L. & Meijer, J. H. Long term effects of sleep deprivation on the mammalian circadian pacemaker. Sleep 30, 257–262 (2007).
Moller-Levet, C. S. et al. Effects of insufficient sleep on circadian rhythmicity and expression amplitude of the human blood transcriptome. Proc. Natl Acad. Sci. USA 110, E1132–E1141 (2013).
Maret, S. et al. Homer1a is a core brain molecular correlate of sleep loss. Proc. Natl Acad. Sci. USA 104, 20090–20095 (2007).
Archer, S. N. & Oster, H. How sleep and wakefulness influence circadian rhythmicity: effects of insufficient and mistimed sleep on the animal and human transcriptome. J. Sleep. Res. 24, 476–493 (2015).
Dallmann, R., Viola, A. U., Tarokh, L., Cajochen, C. & Brown, S. A. The human circadian metabolome. Proc. Natl Acad. Sci. USA 109, 2625–2629 (2012).
Jan, M., Gobet, N., Diessler, S., Franken, P. & Xenarios, I. A multi-omics digital research object for the genetics of sleep regulation. Sci. Data 6, 258 (2019).
Franken, P. et al. NPAS2 as a transcriptional regulator of non-rapid eye movement sleep: genotype and sex interactions. Proc. Natl Acad. Sci. USA 103, 7118–7123 (2006).
Mang, G. M. & Franken, P. Genetic dissection of sleep homeostasis. Curr. Top. Behav. Neurosci. 25, 25–63 (2015).
Franken, P. A role for clock genes in sleep homeostasis. Curr. Opin. Neurobiol. 23, 864–872 (2013).
Skeldon, A. C., Dijk, D. J. & Derks, G. Mathematical models for sleep-wake dynamics: comparison of the two-process model and a mutual inhibition neuronal model. PLoS ONE 9, e103877 (2014).
Dijk, D. J. & Skeldon, A. in Principles and Practice of Sleep Medicine, Seventh Edition (ed. Roth Kryger, G. D.) 390–406 (Elsevier, 2022).
Naylor, E. et al. The circadian clock mutation alters sleep homeostasis in the mouse. J. Neurosci. 20, 8138–8143 (2000).
Kopp, C., Albrecht, U., Zheng, B. & Tobler, I. Homeostatic sleep regulation is preserved in mPer1 and mPer2 mutant mice. Eur. J. Neurosci. 16, 1099–1106 (2002).
Hasan, S., van der Veen, D. R., Winsky-Sommerer, R., Dijk, D. J. & Archer, S. N. Altered sleep and behavioral activity phenotypes in PER3-deficient mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 301, R1821–R1830 (2011).
Hasan, S. et al. A human sleep homeostasis phenotype in mice expressing a primate-specific PER3 variable-number tandem-repeat coding-region polymorphism. FASEB J. 28, 2441–2454 (2014).
Viola, A. U. et al. PER3 polymorphism predicts sleep structure and waking performance. Curr. Biol. 17, 613–618 (2007).
Franken, P., Lopez-Molina, L., Marcacci, L., Schibler, U. & Tafti, M. The transcription factor DBP affects circadian sleep consolidation and rhythmic EEG activity. J. Neurosci. 20, 617–625 (2000).
He, Y. et al. The transcriptional repressor DEC2 regulates sleep length in mammals. Science 325, 866–870 (2009).
Mang, G. M. et al. Altered sleep homeostasis in rev-erbalpha knockout mice. Sleep 39, 589–601 (2016).
Gizowski, C. & Bourque, C. W. The neural basis of homeostatic and anticipatory thirst. Nat. Rev. Nephrol. 14, 11–25 (2018).
Gizowski, C., Zaelzer, C. & Bourque, C. W. Clock-driven vasopressin neurotransmission mediates anticipatory thirst prior to sleep. Nature 537, 685–688 (2016).
Borbély, A. A. in Functional States of the Brain: Their Determinants (eds. Koukou, M., Lehmann D. & Angst, J.) 151–161 (Elsevier, 1980).
Borbely, A. The two-process model of sleep regulation: deginnings and outlook. J. Sleep. Res. 31, e13598 (2022).
Tononi, G. & Cirelli, C. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron 81, 12–34 (2014). This article provides a comprehensive review of the evidence that sleep serves synaptic homeostasis.
Benington, J. H. & Heller, H. C. Restoration of brain energy metabolism as the function of sleep. Prog. Neurobiol. 45, 347–360 (1995).
DiNuzzo, M. & Nedergaard, M. Brain energetics during the sleep-wake cycle. Curr. Opin. Neurobiol. 47, 65–72 (2017).
Nakao, M., McGinty, D., Szymusiak, R. & Yamamoto, M. Thermoregulatory model of sleep control: losing the heat memory. J. Biol. Rhythm. 14, 547–556 (1999).
Harding, E. C., Franks, N. P. & Wisden, W. Sleep and thermoregulation. Curr. Opin. Physiol. 15, 7–13 (2020).
Alfonsa, H. et al. Intracellular chloride regulation mediates local sleep pressure in the cortex. Nat. Neurosci. 26, 64–78 (2023).
Nedergaard, M. & Goldman, S. A. Glymphatic failure as a final common pathway to dementia. Science 370, 50–56 (2020).
Acknowledgements
This publication and some of the research highlighted by P.F. was supported by the Swiss National Science Foundation (grants CRSII3_136201, 31003A_173182, 310030B_192805 and 310030_214847) and the State of Vaud, Switzerland. Research by D.-J.D. is supported by the UK Dementia Research Institute, which receives its funding from DRI Ltd, the UK Medical Research Council, Alzheimer’s Society and Alzheimer’s Research UK. The authors thank S. Archer for comments on the manuscript, and A. C. Skeldon for preparing Fig. 4c.
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to the preparation of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interest.
Peer review
Peer review information
Nature Reviews Neuroscience thanks Chiara Cirelli, Marcos Frank and Frank Scheer for their contribution to the peer review of this article.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Franken, P., Dijk, DJ. Sleep and circadian rhythmicity as entangled processes serving homeostasis. Nat. Rev. Neurosci. 25, 43–59 (2024). https://doi.org/10.1038/s41583-023-00764-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41583-023-00764-z
This article is cited by
-
Differential effects of the stress peptides PACAP and CRF on sleep architecture in mice
NPP—Digital Psychiatry and Neuroscience (2024)