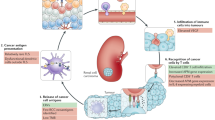
Abstract
Anti-PD1 and anti-PDL1 monotherapies have shown clinical efficacy in stage IV urothelial cancer and are integrated into current clinical practice. However, only a small number of the patients treated with single-agent checkpoint blockade experience an antitumour response. Insufficient priming or inhibitory factors in the tumour immune microenvironment might have a role in the lack of response. CTLA4 is an inhibitory checkpoint on activated T cells that is being studied as a therapeutic target in combination with anti-PD1 or anti-PDL1 therapies in advanced urothelial cancer. In locally advanced urothelial cancer, this combination approach has shown encouraging antitumour effects when administered pre-operatively. We believe that the presence of pre-existing intratumoural T cell immunity is not a prerequisite for response to combination therapy and that the additional value of CTLA4 blockade might involve the broadening of peripheral T cell priming, thereby transforming immunologically cold tumours into hot tumours.
Key points
-
CTLA4 blockade enhances the influx of CD8+ T cells towards the tumour.
-
The addition of anti-CTLA4 to anti-PD1 or anti-PDL1 could be particularly beneficial for immunologically ‘cold’ urothelial tumours, owing to its potential to broaden peripheral T cell priming.
-
Existing results require validation, but data suggest that the optimal dose for ipilimumab in combination with PD1 or PDL1 blockade in urothelial cancer is 3 mg/kg, as opposed to ipilimumab 1 mg/kg in other malignancies.
-
Results of phase III trials with first-line CTLA4 blockade are expected in the near future and could potentially change the treatment landscape of metastatic urothelial cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 (2018).
Stein, J. P. et al. Radical Cystectomy in the Treatment of Invasive Bladder Cancer: Long-Term Results in 1,054 Patients. J. Clin. Oncol. 19, 666–675 (2001).
National Cancer Institute. Surveillance epidemiology and end results program, cancer stat facts: bladder cancer. NIH http://seer.cancer.gov/statfacts/html/urinb.html (2023).
Witjes, J. A. et al. European Association of Urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2020 guidelines. Eur. Urol. 79, 82–104 (2021).
Zargar, H. et al. Multicenter assessment of neoadjuvant chemotherapy for muscle-invasive bladder cancer. Eur. Urol. 67, 241–249 (2015).
Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur. Urol. 48, 202–206 (2005).
Pfister, C. et al. Randomized phase III trial of dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin, or gemcitabine and cisplatin as perioperative chemotherapy for patients with muscle-invasive bladder cancer. Analysis of the GETUG/AFU V05 VESPER trial secondary endpoints: chemotherapy toxicity and pathological responses. Eur. Urol. 79, 214–221 (2021).
Powles, T. et al. Bladder cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann. Oncol. 33, 244–258 (2022).
Freeman, G. J. et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 192, 1027–1034 (2000).
Powles, T. et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial. Lancet Oncol. 22, 931–945 (2021).
Balar, A. V. et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 18, 1483–1492 (2017).
Galsky, M. D. et al. Randomized double-blind phase II study of maintenance pembrolizumab versus placebo after first-line chemotherapy in patients with metastatic urothelial cancer. J. Clin. Oncol. 38, 1797–1806 (2020).
Bellmunt, J. et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N. Engl. J. Med. 376, 1015–1026 (2017).
Galsky, M. D. et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 395, 1547–1557 (2020).
Balar, A. V. et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 389, 67–76 (2017).
Powles, T. et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet 391, 748–757 (2018).
Galsky, M. D. et al. Nivolumab in patients with advanced platinum-resistant urothelial carcinoma: efficacy, safety, and biomarker analyses with extended follow-up from CheckMate 275. Clin. Cancer Res. 26, 5120–5128 (2020).
Powles, T. et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 21, 1574–1588 (2020).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT02603432 (2023).
Vuky, J. et al. Long-term outcomes in KEYNOTE-052: phase II study investigating first-line pembrolizumab in cisplatin-ineligible patients with locally advanced or metastatic urothelial cancer. J. Clin. Oncol. 38, 2658–2666 (2020).
Powles, T. et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N. Engl. J. Med. 383, 1218–1230 (2020).
Blank, C. U., Haanen, J. B., Ribas, A. & Schumacher, T. N. Cancer immunology. The “cancer immunogram”. Science 352, 658–660 (2016).
van Dijk, N. et al. The cancer immunogram as a framework for personalized immunotherapy in urothelial cancer. Eur. Urol. 75, 435–444 (2019).
Gao, J. et al. Neoadjuvant PD-L1 plus CTLA-4 blockade in patients with cisplatin-ineligible operable high-risk urothelial carcinoma. Nat. Med. 26, 1845–1851 (2020).
van Dijk, N. et al. Preoperative ipilimumab plus nivolumab in locoregionally advanced urothelial cancer: the NABUCCO trial. Nat. Med. 26, 1839–1844 (2020).
Sharma, P. et al. Nivolumab alone and with ipilimumab in previously treated metastatic urothelial carcinoma: CheckMate 032 nivolumab 1 mg/kg plus ipilimumab 3 mg/kg expansion cohort results. J. Clin. Oncol. 37, 1608–1616 (2019).
Lindsten, T. et al. Characterization of CTLA-4 structure and expression on human T cells. J. Immunol. 151, 3489–3499 (1993).
Trowbridge, I. S., Collawn, J. F. & Hopkins, C. R. Signal-dependent membrane protein trafficking in the endocytic pathway. Annu. Rev. Cell Biol. 9, 129–161 (1993).
Chan, D. V. et al. Differential CTLA-4 expression in human CD4+ versus CD8+ T cells is associated with increased NFAT1 and inhibition of CD4+ proliferation. Genes Immun. 15, 25–32 (2014).
Takahashi, T. et al. Immunologic self-tolerance maintained by CD25+CD4+ regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J. Exp. Med. 192, 303–310 (2000).
Wang, X. B. et al. Expression of CTLA-4 by human monocytes. Scand. J. Immunol. 55, 53–60 (2002).
Cilio, C. M., Daws, M. R., Malashicheva, A., Sentman, C. L. & Holmberg, D. Cytotoxic T lymphocyte antigen 4 is induced in the thymus upon in vivo activation and its blockade prevents anti-CD3-mediated depletion of thymocytes. J. Exp. Med. 188, 1239–1246 (1998).
Verhagen, J. et al. CTLA-4 controls the thymic development of both conventional and regulatory T cells through modulation of the TCR repertoire. Proc. Natl Acad. Sci. USA 110, E221–E230 (2013).
Vandenborre, K. et al. Interaction of CTLA-4 (CD152) with CD80 or CD86 inhibits human T-cell activation. Immunology 98, 413–421 (1999).
Wang, C. J. et al. Cutting edge: cell-extrinsic immune regulation by CTLA-4 expressed on conventional T cells. J. Immunol. 189, 1118–1122 (2012).
Chambers, C. A., Sullivan, T. J. & Allison, J. P. Lymphoproliferation in CTLA-4-deficient mice is mediated by costimulation-dependent activation of CD4+ T cells. Immunity 7, 885–895 (1997).
Chambers, C. A., Kuhns, M. S. & Allison, J. P. Cytotoxic T lymphocyte antigen-4 (CTLA-4) regulates primary and secondary peptide-specific CD4+ T cell responses. Proc. Natl Acad. Sci. USA 96, 8603–8608 (1999).
Lenschow, D. J., Walunas, T. L. & Bluestone, J. A. CD28/B7 system of T cell costimulation. Annu. Rev. Immunol. 14, 233–258 (1996).
Egen, J. G. & Allison, J. P. Cytotoxic T lymphocyte antigen-4 accumulation in the immunological synapse is regulated by TCR signal strength. Immunity 16, 23–35 (2002).
Ying, H. et al. Cutting edge: CTLA-4–B7 interaction suppresses Th17 cell differentiation. J. Immunol. 185, 1375–1378 (2010).
Blank, C. U. et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat. Med. 24, 1655–1661 (2018).
Walker, L. S. & Sansom, D. M. Confusing signals: recent progress in CTLA-4 biology. Trends Immunol. 36, 63–70 (2015).
Chen, D. S. & Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 541, 321–330 (2017).
Wherry, E. J. & Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 15, 486–499 (2015).
Blackburn, S. D. et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 10, 29–37 (2009).
Tiwari, A. et al. Towards a consensus definition of immune exclusion in cancer. Front. Immunol. 14, 1084887 (2023).
Bruni, S., Mercogliano, M. F., Mauro, F. L., Cordo Russo, R. I. & Schillaci, R. Cancer immune exclusion: breaking the barricade for a successful immunotherapy. Front. Oncol. 13, 1135456 (2023).
Mariathasan, S. et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554, 544–548 (2018).
Wang, L. et al. EMT- and stroma-related gene expression and resistance to PD-1 blockade in urothelial cancer. Nat. Commun. 9, 3503 (2018).
Bruni, D., Angell, H. K. & Galon, J. The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat. Rev. Cancer 20, 662–680 (2020).
Bruhns, P. et al. Specificity and affinity of human Fcγ receptors and their polymorphic variants for human IgG subclasses. Blood 113, 3716–3725 (2009).
Bruhns, P. Properties of mouse and human IgG receptors and their contribution to disease models. Blood 119, 5640–5649 (2012).
Le Goux, C. et al. Correlation between messenger RNA expression and protein expression of immune checkpoint-associated molecules in bladder urothelial carcinoma: a retrospective study. Urol. Oncol. 35, 257–263 (2017).
Das, R. et al. Combination therapy with anti-CTLA-4 and anti-PD-1 leads to distinct immunologic changes in vivo. J. Immunol. 194, 950–959 (2015).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03036098 (2023).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03682068 (2023).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03871036 (2023).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT04960709 (2023).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT05200988 (2023).
Schumacher, T. N. & Schreiber, R. D. Neoantigens in cancer immunotherapy. Science 348, 69–74 (2015).
Morales, A., Eidinger, D. & Bruce, A. W. Intracavitary bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J. Urol. 116, 180–183 (1976).
Baitsch, L. et al. Extended co-expression of inhibitory receptors by human CD8 T-cells depending on differentiation, antigen-specificity and anatomical localization. PLoS ONE 7, e30852 (2012).
Leach, D. R., Krummel, M. F. & Allison, J. P. Enhancement of antitumor immunity by CTLA-4 blockade. Science 271, 1734–1736 (1996).
Sharma, P. et al. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc. Natl Acad. Sci. USA 104, 3967–3972 (2007).
Carthon, B. C. et al. Preoperative CTLA-4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin. Cancer Res. 16, 2861–2871 (2010).
Sharma, A. et al. Anti-CTLA-4 immunotherapy does not deplete FOXP3+ regulatory T cells (Tregs) in human cancers. Clin. Cancer Res. 25, 1233–1238 (2019).
Selby, M. J. et al. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol. Res. 1, 32–42 (2013).
Selby, M. J. et al. Preclinical development of ipilimumab and nivolumab combination immunotherapy: mouse tumor models, in vitro functional studies, and cynomolgus macaque toxicology. PLoS ONE 11, e0161779 (2016).
Quezada, S. A., Peggs, K. S., Curran, M. A. & Allison, J. P. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J. Clin. Invest. 116, 1935–1945 (2006).
Tarhini, A. A. et al. Immune monitoring of the circulation and the tumor microenvironment in patients with regionally advanced melanoma receiving neoadjuvant ipilimumab. PLoS ONE 9, e87705 (2014).
Ribas, A. et al. Intratumoral immune cell infiltrates, FoxP3, and indoleamine 2,3-dioxygenase in patients with melanoma undergoing CTLA4 blockade. Clin. Cancer Res. 15, 390–399 (2009).
Huang, R. R. et al. CTLA4 blockade induces frequent tumor infiltration by activated lymphocytes regardless of clinical responses in humans. Clin. Cancer Res. 17, 4101–4109 (2011).
Simpson, T. R. et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J. Exp. Med. 210, 1695–1710 (2013).
Bulliard, Y. et al. Activating Fc γ receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies. J. Exp. Med. 210, 1685–1693 (2013).
Du, X. et al. A reappraisal of CTLA-4 checkpoint blockade in cancer immunotherapy. Cell Res. 28, 416–432 (2018).
Arce Vargas, F. et al. Fc Effector function contributes to the activity of human anti-CTLA-4 antibodies. Cancer Cell 33, 649–663.e644 (2018).
Romano, E. et al. Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patients. Proc. Natl Acad. Sci. USA 112, 6140–6145 (2015).
Liakou, C. I. et al. CTLA-4 blockade increases IFNγ-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc. Natl Acad. Sci. USA 105, 14987–14992 (2008).
Cascone, T. et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat. Med. 27, 504–514 (2021).
Cretney, E., Kallies, A. & Nutt, S. L. Differentiation and function of Foxp3+ effector regulatory T cells. Trends Immunol. 34, 74–80 (2013).
Dieu-Nosjean, M. C., Goc, J., Giraldo, N. A., Sautès-Fridman, C. & Fridman, W. H. Tertiary lymphoid structures in cancer and beyond. Trends Immunol. 35, 571–580 (2014).
Schrama, D. et al. Immunological tumor destruction in a murine melanoma model by targeted LTα independent of secondary lymphoid tissue. Cancer Immunol. Immunother. 57, 85–95 (2008).
Bruno, T. C. New predictors for immunotherapy responses sharpen our view of the tumour microenvironment. Nature 577, 474–476 (2020).
Helmink, B. A. et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 577, 549–555 (2020).
Cabrita, R. et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 577, 561–565 (2020).
O’Melia, M. J., Manspeaker, M. P. & Thomas, S. N. Tumor-draining lymph nodes are survival niches that support T cell priming against lymphatic transported tumor antigen and effects of immune checkpoint blockade in TNBC. Cancer Immunol. Immunother. 70, 2179–2195 (2021).
Sato, Y. et al. CD4+ T cells induce rejection of urothelial tumors after immune checkpoint blockade. JCI Insight https://doi.org/10.1172/jci.insight.121062 (2018).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
Larkin, J. et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 373, 23–34 (2015).
Ali, S. et al. European Medicines Agency extension of indication to include the combination immunotherapy cancer drug treatment with nivolumab (Opdivo) and ipilimumab (Yervoy) for adults with intermediate/poor-risk advanced renal cell carcinoma. ESMO Open 5, e000798 (2020).
Vellanki, P. J. et al. FDA approval summary: nivolumab with ipilimumab and chemotherapy for metastatic non-small cell lung cancer, a collaborative Project Orbis review. Clin. Cancer Res. 27, 3522–3527 (2021).
Doki, Y. et al. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N. Engl. J. Med. 386, 449–462 (2022).
Baas, P. et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet 397, 375–386 (2021).
Lenz, H. J. et al. First-line nivolumab plus low-dose ipilimumab for microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: the phase II CheckMate 142 study. J. Clin. Oncol. 40, 161–170 (2022).
Yau, T. et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol. 6, e204564 (2020).
Sharma, P. et al. Efficacy and tolerability of tremelimumab in locally advanced or metastatic urothelial carcinoma patients who have failed first-line platinum-based chemotherapy. Clin. Cancer Res. 26, 61–70 (2020).
US National Library of Medicine ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03557918 (2023).
Buchbinder, E. I. & Desai, A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am. J. Clin. Oncol. 39, 98–106 (2016).
Sharma, P. et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol. 17, 1590–1598 (2016).
Grimm, M. O. et al. Tailored immunotherapy approach with nivolumab with or without ipilimumab in patients with advanced transitional cell carcinoma after platinum-based chemotherapy (TITAN-TCC): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 24, 347–359 (2023).
Sharma, P. et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 18, 312–322 (2017).
Massard, C. et al. Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J. Clin. Oncol. 34, 3119–3125 (2016).
Galsky, M. D. et al. Phase 2 trial of gemcitabine, cisplatin, plus ipilimumab in patients with metastatic urothelial cancer and impact of DNA damage response gene mutations on outcomes. Eur. Urol. 73, 751–759 (2018).
Galsky, M. D. et al. A phase III, randomized, open-label, multicenter, global study of first-line durvalumab plus standard of care (SoC) chemotherapy and durvalumab plus tremelimumab, and SoC chemotherapy versus SoC chemotherapy alone in unresectable locally advanced or metastatic urothelial cancer (NILE). J. Clin. Oncol. 39, TPS504–TPS504 (2021).
Heath, E. I. & Rosenberg, J. E. The biology and rationale of targeting nectin-4 in urothelial carcinoma. Nat. Rev. Urol. 18, 93–103 (2021).
Rosenberg, J. LBA73 — Study EV-103 Cohort K: antitumor activity of enfortumab vedotin (EV) monotherapy or in combination with pembrolizumab (P) in previously untreated cisplatin-ineligible patients (pts) with locally advanced or metastatic urothelial cancer (la/mUC). Ann. Oncol. https://doi.org/10.1016/annonc/annonc1089 (2022).
Nicolò, E. et al. Combining antibody-drug conjugates with immunotherapy in solid tumors: current landscape and future perspectives. Cancer Treat. Rev. 106, 102395 (2022).
Versluis, J. M., Long, G. V. & Blank, C. U. Learning from clinical trials of neoadjuvant checkpoint blockade. Nat. Med. 26, 475–484 (2020).
Stimson, C. J. et al. Early and late perioperative outcomes following radical cystectomy: 90-day readmissions, morbidity and mortality in a contemporary series. J. Urol. 184, 1296–1300 (2010).
Necchi, A. et al. Updated results of PURE-01 with preliminary activity of neoadjuvant pembrolizumab in patients with muscle-invasive bladder carcinoma with variant histologies. Eur. Urol. 77, 439–446 (2020).
Powles, T. et al. Clinical efficacy and biomarker analysis of neoadjuvant atezolizumab in operable urothelial carcinoma in the ABACUS trial. Nat. Med. 25, 1706–1714 (2019).
Necchi, A. et al. Pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle-invasive urothelial bladder carcinoma (PURE-01): an open-label, single-arm, phase II study. J. Clin. Oncol. 36, 3353–3360 (2018).
van Dorp, J. et al. High- or low-dose preoperative ipilimumab plus nivolumab in stage III urothelial cancer: the phase 1B NABUCCO trial. Nat. Med. https://doi.org/10.1038/s41591-022-02199-y (2023).
Rosenberg, J. E. et al. Study EV-103: preliminary durability results of enfortumab vedotin plus pembrolizumab for locally advanced or metastatic urothelial carcinoma. J. Clin. Oncol. 38, 441–441 (2020).
Powles, T. LP166: VOLGA: Results from the phase 3 safety run-in with durvalumab (D) + tremelimumab (T) + enfortumab vedotin (EV) for cisplatin-ineligible muscle-invasive bladder cancer (MIBC) (14th European Multidisciplinary Congress on Urological Cancers, 2022).
Catto, J. W. F. et al. Effect of robot-assisted radical cystectomy with intracorporeal urinary diversion vs open radical cystectomy on 90-day morbidity and mortality among patients with bladder cancer: a randomized clinical trial. J. Am. Med. Assoc. 327, 2092–2103 (2022).
Vetterlein, M. W. et al. Improving estimates of perioperative morbidity after radical cystectomy using the European Association of Urology Quality Criteria for standardized reporting and introducing the comprehensive complication index. Eur. Urol. 77, 55–65 (2020).
Clements, M. B. et al. Health-related quality of life for patients undergoing radical cystectomy: results of a large prospective cohort. Eur. Urol. 81, 294–304 (2022).
James, N. D. et al. Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N. Engl. J. Med. 366, 1477–1488 (2012).
Chan, K. K. & Bass, A. R. Autoimmune complications of immunotherapy: pathophysiology and management. BMJ 369, m736 (2020).
Khoja, L., Day, D., Wei-Wu Chen, T., Siu, L. L. & Hansen, A. R. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann. Oncol. 28, 2377–2385 (2017).
Rose, L. M. et al. Incidence of skin and respiratory immune-related adverse events correlates with specific tumor types in patients treated with checkpoint inhibitors. Front. Oncol. 10, 570752 (2020).
Boutros, C. et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat. Rev. Clin. Oncol. 13, 473–486 (2016).
Xu, C. et al. Comparative safety of immune checkpoint inhibitors in cancer: systematic review and network meta-analysis. Br. Med. J. 363, k4226 (2018).
Wolchok, J. D. et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 11, 155–164 (2010).
Ascierto, P. A. et al. Ipilimumab 10 mg/kg versus ipilimumab 3 mg/kg in patients with unresectable or metastatic melanoma: a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 18, 611–622 (2017).
Hammers, H. J. et al. Safety and efficacy of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma: the CheckMate 016 study. J. Clin. Oncol. 35, 3851–3858 (2017).
Hellmann, M. D. et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol. 18, 31–41 (2017).
Rizvi, N. A. et al. Durvalumab with or without tremelimumab vs standard chemotherapy in first-line treatment of metastatic non-small cell lung cancer: the MYSTIC phase 3 randomized clinical trial. JAMA Oncol. 6, 661–674 (2020).
Leighl, N. B. et al. CCTG BR34: a randomized phase 2 trial of durvalumab and tremelimumab with or without platinum-based chemotherapy in patients with metastatic NSCLC. J. Thorac. Oncol. 17, 434–445 (2022).
Chaft, J. et al. Abstract CT113: safety and activity of second-line durvalumab + tremelimumab in non-squamous advanced NSCLC. Cancer Res 78 (13_Supplement), CT113 (2018).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT02542293 (2023).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT02453282 (2023).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03164616 (2023).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT02352948 (2023).
Rozeman, E. A. et al. Identification of the optimal combination dosing schedule of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma (OpACIN-neo): a multicentre, phase 2, randomised, controlled trial. Lancet Oncol. 20, 948–960 (2019).
Vos, J. L. et al. Neoadjuvant immunotherapy with nivolumab and ipilimumab induces major pathological responses in patients with head and neck squamous cell carcinoma. Nat. Commun. 12, 7348 (2021).
Chalabi, M. et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat. Med. 26, 566–576 (2020).
Author information
Authors and Affiliations
Contributions
C.F.S. researched data for the article. All authors contributed substantially to discussion of the content. C.F.S. wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
M.D.G. declares research funding from Janssen, Merk, Dendreon, Novartis, Bristol-Myers Squibb, AZ and Genentech/Roche; consulting fees from BioMotiv, Janssen, Merk, Dendreon, GlaxoSmithKline, Lilly, Astellas, Genetech, BMS, Novartis, Pfizer, EMD Serono, AZ, Seattle Genetics, Incyte, Aileron Therapeutics, Dracen, Inovio Pharmaceuticals and NuMab; and stock from Rapt Therapeutics. M.S.v.d.H. declares the following competing interests: research funding from Bristol-Myers Squibb, AstraZeneca, Roche and 4SC; and consultancy fees from Bristol-Myers Squibb, Merck, Roche, AstraZeneca, Seagen, Pfizer and Janssen (all paid to the Netherlands Cancer Institute). C.F.S. declares no competing interests.
Peer review
Peer review information
Nature Reviews Urology thanks David McConkey and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Stockem, C.F., Galsky, M.D. & van der Heijden, M.S. Turning up the heat: CTLA4 blockade in urothelial cancer. Nat Rev Urol 21, 22–34 (2024). https://doi.org/10.1038/s41585-023-00801-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-023-00801-7
This article is cited by
-
Molecular classification of high-grade, muscle-invasive urothelial carcinomas and the relationship with CTLA-4 and PD-L1 expression
Surgical and Experimental Pathology (2023)