Abstract
Shellfish with rigid shells prevent damage to their delicate internal cores, and their soft bonding muscles drive the opening and closing of the shells. This synergism of rigid and soft materials provides shellfish with unique environmental adaptation. Inspired by the structural characteristics of mussels, a riveting layer was introduced into hydrogel-plastic hybrids for bonding hydrogel networks and plastic substrates. The bonding strength of the hydrogel on the polypropylene (PP) substrate was approximately 1.52 MPa, and the interface toughness reached 1450 J m−2. Furthermore, the integration of plastics and microscale hydrogels, as well as abscised or prefabricated hydrogels, could also be fabricated through the same process. By using this strategy, a hydrogel-plastic hybrid-based device with temperature responsiveness and scratch resistance was fabricated and could mimic the basic activities of mussels. This work improves the functional materials used in programmable engineering systems and could facilitate the construction of intelligent robots.
Similar content being viewed by others
Introduction
With the rapid development of advanced technology, robots have been used in various harsh scenarios, covering ultralow temperatures, the deep sea, and universe space1,2,3. Currently, functional plastics with the advantages of light weight, solvent tolerance and high mechanical strength have become one of the most important building block materials for the preparation of robots in addition to metal materials4,5. However, these conventional robots are commonly rigid and can bend and straighten only around fixed points, restricting their application in dynamic, unstructured, or confined environments. It is still a major challenge for the advancement of robots to perform safe interactions and adaptative functions in unpredictable environments while maintaining high impact and scratch resistance.
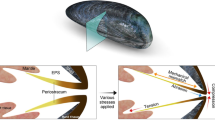
After ten thousand years of natural selection and evolution, organisms possess the perfect organic‒inorganic interactivity by combining soft and hard materials, such as the gelatinous adductor muscle with its shell, human Achilles tendon with the bone, and sea cucumber skin with its inner bone. Specifically, it has been reported that the mussel relies on the glue protein in its body to form a solid bond between its hydrogel-like muscle fibers and the shell, which enables the mussel to protect against the damage caused by external forces6,7. Additionally, these unique structures provide them with environmental adaptability, exhibiting stimulus-responsive properties8. According to previous studies, the muscle fiber constructed by a protein network does not attach to the shell directly but uses the matrix proteins as the riveting points to bridge the gap between their chemical compositions (Fig. 1a). In fact, gel materials, as emerging flexible materials, have attracted great attention in mechanical engineering owing to their various structural possibilities and tunable physical and chemical properties9,10,11. Additionally, gels exhibit shape memory12,13, multistimulus responsiveness14,15, and programmable modulus variability16,17, which are considered to be promising materials for intelligent machineries. Therefore, hydrogel-plastic hybrids with both soft and rigid features could be fabricated by constructing a transition layer with similar bonding modes to the gel network, overcoming the obstacle in environmental adaptation.
a Bonding of the muscle fiber to the shell using the matrix proteins as the riveting points to bridge the gap of the chemical compositions. b Epitaxially grown hydrogel network from the riveting point to form hybrids with high bonding strength. c S 2p XPS spectra of PVC substrates before and after polymerization with DMAPS precursor solution. d P 2p XPS spectra of the substrates before and after polymerization with HEMAP precursor solution.
Compared to the hybrid methods of designing unique substrates18, we achieved tough bonding between soft hydrogels and different kinds of solid plastics through surface modification. In this work, acryloyl chloride with double bonds, which exhibits a similar chemical structure to hydrogel monomers, was selected to modify the surface of functional plastics, constructing the polymerization-active riveting layer. Subsequently, the acryloyl functional groups formed molecular brushes with the monomers in the solution under in situ free radical-initiated polymerization, establishing a stable cross-linked interface. This process played a dominant role in establishing a strong bonding strength between the soft hydrogels and solid plastic substrates. Furthermore, the chain entanglement between the hydrogel and the surface-tethered polymer also contributed to the overall bonding strength. Through this method, we successfully achieved strong bonding between hydrogels and various functional plastics, including polypropylene (PP), polyvinyl chloride (PVC), and acrylonitrile butadiene styrene (ABS). For instance, the integration strength of the hydrogel on PP was more than 1.52 MPa, while the interface toughness reached 1450 J m−2. More importantly, this combined strategy could be used for the repair of damaged interfaces, as well as the integration of prefabricated gels with the designed morphology. Furthermore, we prepared a signal-responsive device based on hydrogel-plastic hybrids with temperature responsiveness and scratch resistance. These functional soft-rigid composites could mimic the opening and closing state of mussels in response to changes in temperature and protect the soft core from scratch damage, demonstrating their practical possibilities for programmable engineering systems. Our work provides a new approach to integrate hydrogels and functional plastic materials, facilitating a broader design platform for advanced intelligent robots.
Materials and methods
Materials and chemicals
Acrylic acid (AA), dimethyl sulfoxide (DMSO), acrylamide (AAm), hydrochloric acid (HCl, 37 wt.%) and H2O2 were purchased from Sinopharm Chemical Reagent Co., Ltd., China. Triethylamine, dioxane, 3-[[2-(methacryloyloxy)ethyl]dimethylammonio]propanesulfonate (DMAPS) and acryloyl chloride were purchased from Aladdin. N,N’-methylenebis(acrylamide) (MBAA), sodium chloride (NaCl), 2-hydroxyethyl methacrylate phosphate (HEMAP) and glutaraldehyde aqueous solution (50 wt.%) were purchased from Sigma‒Aldrich. 2,2-Diethoxyacetophenone (DEAP) and 2-phenoxyethyl acrylate (PEA) were purchased from J&K Chemicals. Poly(ethylene glycol) diacrylate (PEGDA-700, Mn = 700) was purchased from Macklin Biochemical Co., Ltd. Polyvinyl alcohol (PVA, degree of hydrolysis 99%) was purchased from Sinopec Sichuan Vinylon Works. Deionized water, which was used for aqueous solutions, was prepared by a Milli-Q system. All reagents were directly used without any purification. PVC substrates with a thickness of 180 μm, ABS substrates with a thickness of 200 μm, and PP substrates with a thickness of 200 μm were obtained from Zhicheng Plastic Industry Co., Ltd.
Treatment of the plastic substrates
All plastics were cut into 3 × 3 cm pieces and thoroughly rinsed with ethanol to remove any dust or contaminants. Plasma treatment was applied using a low-temperature plasma surface treatment machine (SY-DT03, OPS Plasma, China). The plasma power was 200 W, the exposure time was 10 min, and the distance between the plasma nozzle and the substrate surface was approximately 20 cm. Subsequently, the plastics were soaked in a 30 wt% H2O2 solution. After drying the surfaces, they were immersed in a solution composed of 1.000 g acryloyl chloride, 3.000 g triethylamine and 20.000 g dioxane for 15 min.
Synthesis of the hydrogel-plastic hybrids
Initially, 100 μL DEAP was added to 5.0 mL ethyl acetate to obtain an initiator solution. Twenty microliters of the initiator solution was added dropwise onto the surface of the plastic substrates measuring 3 × 3 cm. After allowing the ethyl acetate to evaporate, a DEAP coating was formed on the glass slide.
For the peeling measurements, poly(DMAPS-co-AA) hydrogel was selected. First, 0.900 g DMAPS, 3.600 g AA and 5.5 mL 4 mol L−1 NaCl were mixed to form the precursor solution. The uniform solution was used to fill in the mold placed on the treated plastics covered with DEAP (Fig. S1). After, the hybrids were fabricated through UV-initiated polymerization (PL-LED100, 365 nm). The samples were made into a rectangle with a size of 25 × 76 mm. For the samples of tensile tests, PVA/AAm double-network hydrogel was selected. A total of 30.000 g AAm, 0.070 g MBAA, 500 μL HCl, 25.000 g PVA aqueous solution (10 wt.%), 1.800 g glutaraldehyde aqueous solution (50 wt.%) and 42.630 g deionized water were mixed to form the precursor solution. The solution was then added to the mold (16 × 12 × 2 mm) placed on the treated plastics covered with DEAP. The hybrids were fabricated through UV-initiated polymerization (PL-LED100, 365 nm). In addition, after UV-initiated polymerization, the samples were incubated in a 60 °C oven for 1 h to ensure the complete reaction between PVA and glutaraldehyde. For the samples used to demonstrate bonding and scratch resistance, the PVA/AAm double-network hydrogel was also selected. However, in this case, no glutaraldehyde was added. For the samples used to mimic the joint activities, poly(AA-co-AAm) hydrogel was selected. AA (0.500 g), 4.500 g AAm, 15.000 g deionized water and 0.025 g MBAA were mixed to form the precursor solution. For fabrication of the artificial plastic shell, poly(AAm-co-PEA) was selected. This temperature-responsive hydrogel was fabricated as reported in previous work19. PEA (7.391 g), AAm (1.815 g), and PEGDA-700 (0.023 g) were dissolved in 7.7 mL of DMSO to form the precursor solution.
Characterization
The microscale morphology was obtained by an atomic force microscope (AFM, SPM-8000, Shimadzu) and a white light interferometer (Bruker Contour Elite I). The elemental analysis was recorded via an X-ray photoelectron spectrometer (ThermoFisher Scientific ESCALAB 250Xi). The mechanical properties were measured by an electrical universal material testing machine with a 500 N load cell (EZ-Test, SHIMADZU). The crosshead velocity was kept at 5 mm min−1 for tensile measurement. The tensile test was performed by a universal testing machine in the range of 1000 N, and the pulling rate was 10 mm min−1 in air. The peeling measurement test was performed by a tensile-compressive tester (M5-2), and the pulling rate was 40 mm min−1 in air.
Results and discussion
Fabrication of hydrogel-plastic hybrids
Based on the bonding mechanism of adductor-muscle, we constructed a riveting layer with the ability to bind with both substrate and gel networks. Figure S1 shows the chemical grafting process of the riveting layer. First, the surface of the plastic was activated by the directional plasma, and reactive hydroxyl groups were formed after the hydrogen peroxide treatment. Then, the carbon‒carbon double bonds that could be copolymerized with the monomers were riveted on the surface by using acryloyl chloride (AC). Subsequently, in situ free radical-initiated polymerization was used to ensure polymer chain propagation starting from the riveting point. As shown in Fig. 1b, such epitaxially grown hydrogel networks from the substrate formed stable chemical cross-links, which could overcome the weak interfacial bonding originating from the surface instability of the hydrogel. To verify the mechanisms of in situ free radical-initiated polymerization epitaxially grown, Fourier transform infrared spectroscopy (FTIR) was performed to investigate the changes in the chemical composition and microstructure of the substrate surface. As Fig. S2 shows, the peak associated with the C = C bond at 1640 cm−1 was intensified after acryloyl chloride modification and weakened after UV-initiated polymerization. This observation indicated the anchoring and consumption processes20,21. Additionally, we performed XPS measurements on PVC samples that were polymerized using DMAPS and 2-hydroxyethyl methacrylate phosphate (HEMAP) precursor solutions. In Fig. 1c, the PVC sample polymerized with DMAPS precursor solution exhibited two peaks in the S 2p region at approximately 167 eV and 168 eV. These peaks corresponded to the characteristic energy levels of the covalently bonded sulfonic acid group22. Furthermore, the PVC sample polymerized with HEMAP precursor solution showed an additional peak in the P 2p region (Fig. 1d). This observation confirmed the successful grafting of DMAPS and HEMAP polymer brushes onto the PVC substrate. The construction of the riveting polymer brush (polyacrylamide) on the PVC substrate was confirmed by the AFM images (Fig. S3). This dense nanostructure indicated the high density of riveting sites, as well as the efficient interfacial radical transfer reaction between the riveting site and hydrogel monomers.
Strong bonding between the soft hydrogels and solid plastics
The bonding strength between soft hydrogels and solid plastic substrates is one of the key factors in evaluating the stability and reliability of hydrogel-plastic hybrids23,24,25,26. Here, peeling measurements were carried out to calculate the interfacial toughness. In general, the interactive force between the hydrogel and the plastic with low surface energy was weak. As shown in Fig. S4 and Supplementary Movie S1, the hydrogel constructed on the untreated plastic substrate easily detached in the peeling process, showing almost no interfacial fracture toughness against the external force. Figure 2a shows the peeling process of hydrogel-plastic hybrids; a large deformation of the hydrogel in both the interface and bulk was observed. Theoretically, the interfacial cracks could kink and propagate in the hydrogel network, which was relatively brittle, dissipating mechanical energy (ΓD). Therefore, the interface strength tested by the peeling measurement was the sum of the intrinsic work of bonding (Γ0) and the dissipated mechanical energy of the hydrogel (ΓD), which could be expressed as Γ = ΓD + Γ0 (1)27. To avoid gel fragmentation in the peeling process, a poly(DMAPS-co-AA) hydrogel with high ductility was selected. Figure 2b shows the calculated peeling forces per width of the hydrogel-plastic hybrids. All peeling curves exhibited the same trend; an increase in the peeling force produced rapid growth, followed by a flattening of the curves at a high level. Additionally, the peeling forces between the hybrids based on different plastics, such as PP, PVC and ABS, were almost the same, which demonstrated the good generality of our strategy. Moreover, this similarity indicated that the bonding strength between the hydrogel and the plastics was higher than the fracture strength of the hydrogel. When the peeling force reached the fracture value of the hydrogel network, the crack began to propagate along the bulk hydrogel near the bonding interface. As shown in Fig. 2c, the average interfacial toughnesses of the hydrogel-plastic hybrids were all over 1450 J m−2.
a Optical images of the peeling process; the scale bar is 0.5 cm. b Curves of the peeling force per width versus displacement for the various types of hydrogel-plastic hybrids. c Measured interfacial toughness values of the hybrids. d Digital picture of the tensioning process; the scale bar is 0.5 cm. The inset shows its original state. e Tensile stress of the hybrids based on ABS, PVC and PP. f Statistical tensile stress of the hybrids.
As shown in Fig. 2d, tensile tests were performed to provide further insight into the bonding strength of the hybrids. In this experiment, the ductility of the hydrogel did not have an immediate effect on the results, such as the peeling measurement. Therefore, the PVA/AAm double-network hydrogel with high strength and modulus (Fig. S5) was beneficial for predicting the real interfacial toughness. Figure 2e shows the stress‒strain curves of the hybrids with PP, PVC and ABS substrates. The tensile force on the bonding interface per unit area rapidly increased with the tensile strain, and its sudden fracture occurred when the force reached a specific value. As shown in Fig. S6, the samples constructed using the hydrogels with high strength represented the interfacial fracture. In contrast, the samples prepared by hydrogels with weak mechanical strength exhibited fracture on the hydrogel network after separation. These results indicated that the actual bonding strengths of the hydrogel-plastic hybrids could be obtained with these measurements. As shown in Fig. 2f, the PP-based and ABS-based hybrids have similar average interfacial strengths, which were 1.52 and 1.46 MPa, respectively. However, the interfacial strength of the hybrids based on PVC was 1.13 MPa, which was near 75% of the others. This could be caused by the different polymer chains of the plastics, which were related to the formation of radicals on the substrate surface under plasma treatment28 and led to the distinct density of the riveting sites.
With the development and promotion of robots with various scales, the macroscopic integration of soft and rigid materials cannot address all of the required conditions. For example, hydrogels with biomimetic micro/nanostructures could be used for environmental signal detection and motion monitoring, and micron-scale gel-based ionic circuits could be used in bioionic signal transmission. Thus, we further evaluated the interfacial bonding behaviors of micron-scale hydrogels on the plastic substrates. As shown in Fig. S7, a micron-scale trapezoid with a top width of 70 μm, a bottom width of 90 μm and a height of 25 μm was successfully constructed on the plastic substrates. Moreover, three trapezoidal hydrogels were arranged in the field of view according to the design, and all structures remained intact after demolding due to interfacial bonding. This would provide a new concept and preparation solution for the design and application of gel-based microcircuits.
Although the soft and weak hydrogel components could be protected by rigid plastic, there will still be a large difference in the degree of attrition between them in practical applications. Part replacement is a good way to solve the problem. As shown in Fig. 3a, the hydrogel network, as well as the bonding interface, could be damaged, resulting in the functional failure of the hydrogel-plastic hybrids. At this moment, the plastic substrates could be recycled through secondary treatment, reforming the active riveting sites. The damaged hydrogel would be immersed in the precursor solution to swell the monomers and then placed on the surface of the substrates (emptying the air bubbles in the interface between hydrogel and plastic). Finally, the crack could be healed by forming the second hydrogel network after UV-initiated polymerization. Figure S8 shows the healing process of the hydrogel-plastic hybrids. The interfacial bonding strength of the hybrids was also evaluated by peeling and tensile tests. As shown in Fig. 3b, the peeling forces per width of the PP-based sample reached 796 J m−2. The peeling curves exhibited the same trend as the origin, which demonstrated the binding interaction between the gel and the substrate. The stress‒strain curve also demonstrated successful interfacial bonding, showing a tensile stress of 0.83 MPa (Fig. 3c). Notably, the bonding strength between hydrogels and secondary treatment substrates was relatively lower than the original strength, even though the mechanical strength of the soft network was improved by constructing a double network (DN) hydrogel. This result originated from the lower density of active riveting sites. In the same way, prefabricated hydrogels with specific shapes and sizes could also be bonded on plastic to form functional hybrids, which would facilitate more convenience to their design and application.
Applications of the hydrogel-plastic hybrids originating from tough bonding
The potential applications of our hydrogel-plastic hybrids are shown in Fig. 4. First, the relative position of the hydrogel and substrate was fixed; thus, the hydrogel could be placed in the “armor” constructed by the plastic. In a similar way that the hard shell of the mussel protects the hydrogel-like muscles, the hydrogel core of the hybrid can be protected against external forces, such as scratching and knocking (Fig. 4a). As shown Fig. 4b, the strong bonding between the hydrogel and the plastic makes it possible for the hybrids to mimic joint activities, including stretching, bending and twisting. These hybrids compensate for the restrictions of the small motion range of the plastic and the overly easy deformation of soft hydrogels and could provide the basis for artificial robots to reproduce the function of bones and soft connective tissues (tendon, ligament, etc.). Furthermore, a shape memory hydrogel (poly(AAm-co-PEA)) with temperature responsiveness was used to construct the hydrogel-plastic hybrids19. As Fig. 4c shows, the temperature-responsive gel acts like the adductor muscle that stores and releases energy when it is under external attack (high temperature). By warming the surrounding environment above the temperature of the phase transition (Tm, 37 °C), the elongated “artificial muscle” releases the stored energy and recovers its original shape. As a result, the plastic shell closes under the driving force transmitted through the bonding. Notably, the ability of hybrids to switch between open and closed states repeatedly relies on the responsiveness and cyclic mechanical properties of the hydrogel itself. As demonstrated in the cyclic tensile test (20% strain) (Fig. S9), the stress levels remain in the same order as the original even after 50 cycles, indicating that the hydrogel-plastic hybrid exhibits excellent cyclic durability. These results demonstrate that our hydrogel-plastic hybrids provide a new type of functional material for intelligent robots.
a Scratch resistance of the hybrids, showing the protection of the hydrogel-based core that is a similar to the protection provided by a mussel; the scale bar is 0.5 cm. b Hydrogel bonding as a flexible junction, which can mimic the joint activities of organisms; the scale bar is 0.5 cm. c Closure of the artificial plastic shell under the driving force transmitted through the bonding hydrogel muscle with temperature responsiveness; the scale bar is 1 cm.
Conclusions
In summary, we described a strategy of constructing a polymerization-active riveting layer to achieve strong bonding between hydrogels and plastics, which was similar to the principle found in mussels. This strategy could be used for the fabrication of a wide range of hydrogel-plastic hybrids, including PP-, PVC- and ABS-based composites. Moreover, the integration of plastics and microscale hydrogels, as well as abscised or prefabricated hydrogels, could also be achieved by this strategy, demonstrating its prospects in fine processing. Furthermore, functional hydrogel-plastic hybrids with temperature responsiveness and scratch resistance were successfully prepared, showing the practicality and programmability of our bonding strategy and hybrids. This work highlights the importance and advantages of the integration of hydrogels and plastics in intelligent robots and provides a basis for the applications of ionic sensing, sports rehabilitation, and clinical medicine.
References
Li, G. et al. Self-powered soft robot in the Mariana Trench. Nature 591, 66–71 (2021).
Yuk, H. et al. Hydraulic hydrogel actuators and robots optically and sonically camouflaged in water. Nat. Commun. 8, 14230 (2017).
Liu, Z. et al. Poly(ionic liquid) hydrogel-based anti-freezing ionic skin for a soft robotic gripper. Mater. Horiz. 7, 919–927 (2020).
Polygerinos, P. et al. Soft robotics: review of fluid-driven intrinsically soft devices; manufacturing, sensing, control, and applications in human-robot interaction: review of fluid-driven intrinsically soft robots. Adv. Eng. Mater. 19, 1700016 (2017).
Rasilainen, T., Kirveslahti, A., Nevalainen, P., Suvanto, M. & Pakkanen, T. A. Modification of polypropylene surfaces with micropits and hierarchical micropits/nanodepressions. Surf. Sci. 604, 2036–2042 (2010).
Song, Y. et al. Structural characteristics at the adductor muscle and shell interface in mussel. Appl. Biochem. Biotechnol. 171, 1203–1211 (2013).
Lv, J., Jiang, Y. & Zhang, D. Structural and mechanical characterization of atrina pectinata and freshwater mussel shells. J. Bionic. Eng. 12, 276–284 (2015).
Freeman, A. S., Meszaros, J. & Byers, J. E. Poor phenotypic integration of blue mussel inducible defenses in environments with multiple predators. Oikos 118, 758–766 (2009).
Liu, X. et al. Ingestible hydrogel device. Nat. Commun. 10, 493 (2019).
Sun, T. L. et al. Physical hydrogels composed of polyampholytes demonstrate high toughness and viscoelasticity. Nat. Mater. 12, 932–937 (2013).
Lim, C. et al. Tissue-like skin-device interface for wearable bioelectronics by using ultrasoft, mass-permeable, and low-impedance hydrogels. Sci. Adv. 7, eabd3716 (2021).
Hu, X. et al. Programming temporal shapeshifting. Nat. Commun. 7, 12919 (2016).
Hua, L., Zhao, C., Guan, X., Lu, J. & Zhang, J. Cold-induced shape memory hydrogels for strong and programmable artificial muscles. Sci. China Mater. 65, 2274–2280 (2022).
Fang, J. et al. A shift from efficiency to adaptability: recent progress in biomimetic interactive soft robotics in wet environments. Adv. Sci. 9, 2104347 (2022).
Sun, M. et al. Hydrogel interferometry for ultrasensitive and highly selective chemical detection. Adv. Mater. 30, 1804916 (2018).
Hua, M. et al. Strong tough hydrogels via the synergy of freeze-casting and salting out. Nature 590, 594–599 (2021).
Chen, G. et al. Rapidly and repeatedly reprogrammable liquid crystalline elastomer via a shape memory mechanism. Adv. Mater. 34, 2201679 (2022).
Ma, S. et al. Continuous surface polymerization via Fe(II)-mediated redox reaction for thick hydrogel coatings on versatile substrates. Adv. Mater. 30, 1803371 (2018).
Liang, R. et al. Highly tough hydrogels with the body temperature-responsive shape memory effect. ACS Appl. Mater. Interfaces 11, 43563–43572 (2019).
Wei, D., Zhou, R., Guan, Y., Zheng, A. & Zhang, Y. Investigation on the reaction between polyhexamethylene guanidine hydrochloride oligomer and glycidyl methacrylate. J. Appl. Polym. Sci. 127, 666–674 (2013).
Yamada, T., Shirasaka, K., Noto, M., Kato, H. S. & Kawai, M. Adsorption of unsaturated hydrocarbon moieties on H:Si(111) by grignard reaction. J. Phys. Chem. B 110, 7357–7366 (2006).
Kang, Z. F., Li, E. T. & Neoh, K. G. Surface structures and adhesive-free adhesion characteristics of polyaniline films after modification by graft copolymerization. Macromolecules 30, 3354–3362 (1997).
Yuk, H., Zhang, T., Parada, G. A., Liu, X. & Zhao, X. Skin-inspired hydrogel–elastomer hybrids with robust interfaces and functional microstructures. Nat. Commun. 7, 12028 (2016).
Liu, Q., Nian, G., Yang, C., Qu, S. & Suo, Z. Bonding dissimilar polymer networks in various manufacturing processes. Nat. Commun. 9, 846 (2018).
Li, X.-C., Hao, D.-Z., Hao, W.-J., Guo, X.-L. & Jiang, L. Bioinspired hydrogel–polymer hybrids with a tough and antifatigue interface via one-step polymerization. ACS Appl. Mater. Interfaces 12, 51036–51043 (2020).
Hao, D. et al. Strong anchoring of hydrogels through superwetting–assisted high–density interfacial grafting. Angew. Chem. Int. Ed. 62, e202215034 (2023).
Yuk, H., Zhang, T., Lin, S., Parada, G. A. & Zhao, X. Tough bonding of hydrogels to diverse non-porous surfaces. Nat. Mat. 15, 190–196 (2016).
Lu, W., Cao, T., Wang, Q. & Cheng, Y. Plasma-assisted synthesis of chlorinated polyvinyl chloride (CPVC) using a gas-solid contacting process: plasma–assisted synthesis of CPVC. Plasma Process. Polym. 8, 94–99 (2011).
Acknowledgements
This work was supported by the National Key R&D Program of China (2020YFA0710401), the National Natural Science Foundation of China (52075138, 21905287), and the China Postdoctoral Science Foundation (2022M713226).
Author information
Authors and Affiliations
Contributions
Z.W. proposed the initial idea. Z.W., T.Z. and W.C. carried out the experiments and analyzed the data. All authors were involved in the discussion of the results and wrote the manuscript. X.K. and W.C. supervised the project.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wen, Z., Zhou, T., Xu, Q. et al. Functional hydrogel-plastic hybrids inspired by the structural characteristics of mussels. NPG Asia Mater 15, 42 (2023). https://doi.org/10.1038/s41427-023-00491-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41427-023-00491-y