Abstract
Purpose of Review
Continuous replanting of land with the same or similar plant species can result in the accumulation of harmful soil microbes, which can lead to crop failure. In this review, we explore the influence of constant replanting on the health of short-rotation forestry soil, focusing on the accumulation of deleterious microbes and the decline of beneficial microbes. We also suggest possible practical solutions to address this problem and consider future research that could be conducted to better understand and reduce the build-up of deleterious soil microbes in short-rotation forestry soil.
Recent Findings
Compelling evidence that continuous replanting of the same tree species in short-rotation plantation forestry might contribute to the build-up of deleterious soil microbes is still lacking. However, our assessment of existing soil microbiome data from global short-rotation plantation environments suggests a high risk of an accumulation of harmful microbes and a loss of beneficial microbes in plots that were continually replanted with the same tree species. Based on this evidence, and that from agriculture, we propose further research to acquire a better understanding of the build-up of harmful soil microbes in short-rotation plantation forestry, and suggest crop rotation and intercropping strategies to avoid this malady in the future.
Summary
The accumulation of microbes detrimental to plantation trees and the decline of microbes beneficial to these trees are realistic risks when plantations are continually replanted with the same tree species. Extensive research is necessary to evaluate the impact of short continuous planting rotations on the biodiversity of soil microbes in plantations and to develop strategies that would alleviate the build-up of detrimental microbes.
Similar content being viewed by others
Introduction
Plantation forestry is important to the global economy, and it is increasingly realised that intensively managed plantations have a major role to play in the circular economy by providing sustainable material and replacing fossil-based products [1]. Forests cover about four billion hectares of the world’s land surface, of which about 291 million hectares are planted forests [2]. The commercial forestry sector is continuously expanding due to an increasing global population and demand for forest-based products. Since 1990, the global primary forest area has been steadily decreasing at a rate that is especially high in low-income countries [3]. To compensate for the loss of natural forest resources, nearly all countries are in some way engaged in commercial forestry, which also provides important sources of employment and income [2].
It is unlikely that the global demand for forest-based products will decrease in the future, and this is especially due to the development of numerous novel applications, such as microfibers and composite wood products. Plantation forestry using fast-growing tree species that produce high yields, especially in temperate, tropical and subtropical regions is an important source of these products [4,5]. Hence, forestry companies are continuously seeking sources of rapidly growing genotypes of commercially important tree species with the fit for purpose wood and tolerance to biotic and abiotic stresses [6,7]. These genotypes will substantially reduce rotation periods, and consequently, forest land is likely to be replanted more frequently.
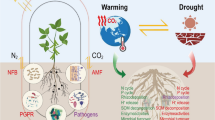
Short-rotation plantation forestry (5–20-year rotation) has the potential to substantially meet the global demand for forest-based products because of the faster accrual of biomass by various species of Eucalyptus, Pinus, Acacia and Populus [8]. However, continuous short rotations of the same or closely related tree species or genotypes may have unintentional ecological consequences (Fig. 1). Competition for plant-derived nutrients allows a large number of microbes to colonize the rhizosphere [9,10], collectively referred to as the rhizobiome, and successive replanting on the same land can lead to a build-up of detrimental microbes that can negatively affect long-term productivity [11]. In a newly established monoclonal plantation, the levels of soil-borne plant pathogens are typically not problematic. Successive short rotations of the same tree genotypes can, however, result in soils with increased loads of deleterious microbes, which could cause crop failure [12, 13••].
Soil health is fostered by the synergistic effect of the physical, chemical and biological properties of the soil. High plant diversity in native vegetation promotes good soil health. However, when vegetation is cleared to set up monoculture plantations, soil health suffers as a result of the low biodiversity of soil microbes triggered by reduced plant diversity, harvesting and other silvicultural activities. This poor soil health in plantations is exacerbated by continuous short rotations of the same or nearly identical tree genera, which deteriorate soil health and promote the build-up of deleterious microbes, causing tree decline
In this review, we evaluate the data from soil microbiome research conducted on short-rotation forest soils to assess the impact of continuous replanting, with an emphasis on detecting evidence for the accumulation of deleterious microbes and the reduction of beneficial ones. Based on this evidence, we recommend directions for further studies required to gain a better understanding of the accumulation of deleterious microbes in short-rotation forestry soils and to minimise the effect of this malady in the future.
Soil Health in Managed Forest Environments
Soil properties, the chemical composition of the plant litter and above-ground vegetation significantly influence the soil microbiome [14•, 15,16,17]. Monoclonal plantations of exotic trees often have poor litter quality and limited diversity in above-ground vegetation. This, along with changes in land use and management techniques can negatively impacts the biodiversity of soil microbes [18,19,20,21]. This is especially concerning because the soil microbiome is responsible for several nutrient cycles and also improves soil fertility [14, 22, 23]. The poor biodiversity of soil microbes can be further intensified through harvesting and subsequent replanting of stands to the same exotic tree species [24,25,26,27] (Fig. 1), ultimately leading to a gradual deterioration of soil health [28, 29]. This, for example, has been documented in a recent study by Guo et al. [30••], in which the authors reported that the transformation of natural broadleaved forests into Cunninghamia lanceolata monocultures resulted in the degradation of soil physiological properties as well as lower diversity and richness of soil microbial communities. Other research that compared the microbial diversity, soil nutrients, and structure of short-rotation tree plantations and natural forests found a comparable pattern [31,32,33].
Soil microbes serve an essential function in forests by recycling nutrients and restoring the physical properties of the soil [9, 11, 34]. Amongst these microbes, fungi act as the primary decomposers and simultaneously improve the physical properties of the soil [9, 35, 36]. In addition, plant symbiotic fungi such as mycorrhizae facilitate nutrient uptake, improve plant resistance to pathogens and enhance stress tolerance [37, 38]. Similarly, soil-inhabiting bacteria such as nitrifying bacteria, mycorrhization helper bacteria [39] and plant growth-promoting rhizobacteria [40] enrich the soil with nutrients, enhance mycorrhizal associations [39] and stimulate plant growth [41].
Soil Health and Multiple Rotations—Lessons from Agriculture and Horticulture
Agricultural and horticultural plantings provide alarming examples of the ‘replanting syndrome’. Two well-documented cases are the take-all disease of wheat and apple replant disease (ARD). The soil-borne fungal pathogen Gaeumannomyces tritici causes take-all disease, one of the most damaging root diseases of wheat [42]. This disease is widespread in temperate wheat-growing regions of the world, causing significant economic losses [43, 44]. G. tritici also infects other cereal crops, including barley, rye and triticale. Usually, this fungus survives saprophytically within plant debris during the intercropping period. Primary infection occurs when the roots of wheat seedlings come into contact with the fungal mycelia. The infection begins with the fungus colonising the root and progresses to the plant’s vascular tissue [45, 46]. Infected plants have distinctive white heads caused by premature ripening of the ears, as well as blackened stem bases [47•]. The severity of this disease increases with continuous replanting [48]. A comparable scenario has been documented in horticultural settings in the form of ARD.
ARD has been reported in almost all apple-growing areas in the world [49, 50•, 51]. Symptoms of this syndrome include stunted tree growth, discoloured roots, reduction of the root mass and necrosis of the root tips. The degree of susceptibility varies greatly among apple genotypes [52]. Nonetheless, symptoms appear soon after planting into continuously replanted soil [50•, 53,54,55]. Apart from apples, various other rosaceous crops are also known to be affected by this disease [56, 57]. ARD is known to be caused by biotic components since fumigation, and other methods of disease control can suppress this syndrome [57, 58]. Several different soil-borne necrotrophic fungi and oomycete pathogens, such as species of Fusarium, Cylindrocarpon, Phytophthora, Pythium and Rhizoctonia, have been recovered from symptomatic trees. Therefore, it appears unlikely that it is caused by a single pathogen, but rather by a pathogen complex [49, 50•, 55, 59,60,61,62,63]. In addition, certain edaphic factors can also increase the severity of the disease, such as soil structure, pH and nutrients [55, 57]. Continuous replanting, for example, alters soil microbial interactions and metabolism [64, 65]. Inefficient litter decomposition can result in the build-up of phenolic compounds such as phlorizin, benzoic acid and vanillic aldehyde [66]. The combined effect of these biochemical processes negatively influences essential soil components such as pH, organic matter, moisture levels and the availability of N, K and P [67] and can adversely affect soil microbes, leading to the development of ARD.
In both the above-mentioned examples, chemical control is expensive, usually ineffective and environmentally unsustainable. Various isolates of G. tritici are known to be resistant to commonly used fungicides [68], and chemical control for ARD is hazardous to the environment and is not sustainable due to the scale of the affected areas [57]. In this regard, disease-resistant crop varieties are often considered as a solution to the problem. Various resistant apple rootstocks have thus been developed, which are partially resistant to ARD [69], but for take-all disease, no disease-resistant wheat cultivars are currently available [44]. For both of these diseases, however, crop rotation is an effective control strategy [44, 48, 57, 58].
Crop rotation involves cultivating different, unrelated plant species sequentially on the same land [70, 71], a strategy that can significantly reduce the build-up of unfavourable microbes [49, 59, 72]. At the same time, crop rotation increases the nutrient levels and physical properties of the soil [48, 73,74,75,76,77]. Such a rotation of crops is especially effective in reducing plant diseases caused by biotrophic pathogens, because these microbes lack saprotrophic life stages, and cannot survive without a living host [78]. The efficacy of crop rotation is substantially less effective in the case of plant pathogens that have a broad host range or that produce thick-walled structures such as sclerotia, chlamydospores or thick-walled oospores, which can overcome unfavourable conditions [79,80].
The selection of an appropriate rotation programme is important for this strategy to be effective [74, 81, 82]. For example, alternating between wheat and legumes is commonly practised to control the take-all disease caused by G. tritici [48]. The legumes restore soil nitrogen and minimize the build-up of pathogen propagules resulting in higher grain yield, while the grains improve the physical structure of the soil providing niches for the survival of beneficial microbes [74, 81, 83, 84]. Similarly, rice is usually rotated with maize and various leguminous crops to improve soil nutrients and structure [85,86,87]. Likewise, potato, an important cash crop, is often rotated with buckwheat, oats, ryegrass or clover to reduce the inoculum of Rhizoctonia solani in the soil [88,89,90,91] (Table 1). These are examples where crop rotation is an effective and sustainable method for improving the health of agricultural soils and, as a result, it is extensively used when growing annual crops. However, for fruit tree orchards, the situation is more complex due to the long lifespans of the plants and the specialized nature of the cropping system.
Evidence for Accumulation of Detrimental Soil Microbes in Plantation soils
Short-rotation plantation forestry plots are often established by clearing land that was previously covered by other vegetation types (Fig. 1). After clearing the previous, in some cases native vegetation, a suitable tree species is selected based on performance under the prevailing conditions in the region, including climate, soil and other factors. These plantations are often continuously replanted to the same genera, every 10–15 years. Recent investigations comparing the community of soil microbes in monoclonal planted forests and neighbouring mixed natural forest areas have revealed that plantation soils harbour higher levels of microbes that are pathogenic to the plantation tree species [92••, 93••, 94••]. In all of these studies, the community composition of microbes associated with plantation soils differed significantly from that of adjacent native forests.
Jimu et al. [92••] compared the community composition of soil fungi associated with exotic Eucalyptus grandis and adjacent woodlands in Zimbabwe. In that study, the soil mycobiota of the E. grandis plantation included fungal taxa from families such as the Davidiellaceae, Mycosphaerellaceae and Teratosphaeriaceae, which include species that are known as Eucalyptus pathogens. Similarly, continuous replanting of many other tree species over several generations has been shown to have deleterious effects on tree health due to disturbance of soil microbial diversity [13, 95••].
Cunninghamia lanceolata is native to China. This tree is planted due to its rapid growth, high yields and the fact that it adapts to a wide range of climatic conditions. There have been reports of replanting problems with the monocultures of this tree. Continuous replanting with C. lanceolata reduces soil fertility and negatively affects the soil microbial community [13••, 30••, 95••]. Xia et al. [12] demonstrated that soil microbial community compositions differ between the first and second rotations of C. lanceolata plantings. For example, an upsurge in Fusarium and Penicillium species was observed during the second rotation. However, the authors could not distinguish between pathogenic and non-pathogenic species of Fusarium. The authors hypothesised that the deterioration of the soil microbial community was likely caused by the continual replanting of the plots (Table 1). To alleviate this problem, C. lanceolata is now often rotated with P. massoniana [96].
Various Phytophthora species, such as P. alticola, P. cinnamomi and P. frigida, are important soil-borne pathogens of plantation trees. Studies in which the community of Phytophthora species were compared between plantations, and natural mixed forests indicated an accumulation of specific Phytophthora species that are pathogenic to the plantation tree species [94••, 97, 98]. They also showed that the species composition of Phytophthora was different from the adjacent mixed natural forests [94••, 98]. The species richness of non-pathogenic Phytophthora species was substantially lower in the plantation soil and roots of non-native plantations trees, E. grandis and Acacia mearnsii, but included those that are known pathogens of these trees [94••, 98] (Table 1).
There is currently very little compelling experimental evidence that continuous planting of the same or nearly similar tree species in short-rotation forestry results in the accumulation of detrimental microbes that cause tree decline. This is due to the lack of long-term research monitoring the accumulation of soil microbes in short-rotation plantation forest plots. However, the research cited above provides evidence that some pathogenic microbes can become more prevalent in short-rotation forest soils. Thus, it cannot be excluded that continuous replanting of the same tree species in a plot already loaded with detrimental microbes, as practised in short-rotation forestry, could allow these pathogens to become more abundant over time, resulting in tree decline (Fig. 1; Table 1).
Evidence for the Reduction of Beneficial Soil Microbes in Plantation Soils
Reduced presence of beneficial microbes, such as saprophytes, mycorrhizae and rhizobia, in plantation soil can also have an adverse impact on plant health. As a result, in short-rotation plantations, the synergistic impact of pathogen accumulation and the decline of beneficial microbes can lead to a deterioration of soil and tree health. Beneficial microbes are fundamentally important in the regulation of soil biogeochemical processes because they are the primary drivers of nutrient cycling and soil quality improvement [99, 100]. Saprophytes, mycorrhizal fungi and rhizobia, for example, play critical roles in decomposing plant biopolymers, as well as promoting nutrient uptake, boosting plant metabolism and increasing disease resistance [9, 101, 102].
Saprotrophic fungi are essential components of the nutrient cycle in terrestrial environments. They are the primary decomposers of plant litter, and their hyphal networks, which spread along the soil-litter interface, represent active routes through which these nutrients are efficiently distributed [103, 104]. Xu et al. [105••] reported that continuous replanting of Eucalyptus can reduce the relative abundance of dominating microbial groups. The authors reported that switching from P. massoniana (coniferous) to Eucalyptus (broadleaf) improved soil fungal colonisation in the early phases (first and second generations). However, subsequent generations negatively impacted the physiochemical properties of the soil and the community diversity of soil microbes. Soil bacterial communities changed from carbon-utilizing to nitrogen-utilizing, whereas the fungal communities shifted from saprophytic and pathogenic to symbiotic. Earlier, Xu et al. [106•] compared the effect of continuous monoculture of Eucalyptus plantations on nutrient levels and microbial biomass (fungi and bacteria) to that of inter-planting Eucalyptus with Manglietia glauca. When compared to monoclonal Eucalyptus plots, interplanting Eucalyptus with M. glauca enhanced soil fertility and increased the number and richness of beneficial fungi and bacteria (Table 1).
Mycorrhizal fungi mobilise nutrients, such as N and P, to the host plant and boost the host’s tolerance to abiotic (drought, salt, heavy metals) and biotic (root pathogens) stress. Eucalyptus species form associations with both ecto and endomycorrhizae. Chen et al. [107•] reported low levels of inoculum of both ectomycorrhizae and endomycorrhizae in plantation soil when documenting the mycorrhizal biodiversity associated with short-rotation Eucalyptus plantations in China over a 2-year period. In contrast, Xu et al. [105••] showed that the relative abundance of mycorrhizal fungi was initially low during the early rotational phase (first and second generation). Later on, when soil fertility declined (third and fourth generation), the abundance of mycorrhizal fungi increased. This trend was similar in a number of other studies conducted on E. grandis [108] and E. saligna [109] (Table 1). However, during the early stages of replanting, when mycorrhizal abundance is minimal in the soil, pathogen abundance can be higher [105•]. As a result, the presence of soil-borne pathogens may have an adverse effect on the future colonisation of Eucalyptus roots by mycorrhizal fungi. For example, as demonstrated with Eucalyptus gomphocephala, the presence of Phytophthora multivora in the soil lowers fine root biomass, resulting in reduced ectomycorrhizal fungal colonisation [110, 111] (Table 1).
Acacia species are important plantation trees in the tropical regions of the world. As leguminous trees, they form symbiotic associations with rhizobia that fix atmospheric nitrogen. de São José et al. [112••] investigated the rhizobial diversity associated with A. mearnsii at multiple sampling sites located in Brazil. The authors reported that the genetic diversity of rhizobial species was higher at sampling sites that were planted for the first time with A. mearnsii, whereas sites that were continuously replanted with A. mearnsii had lower genetic diversity of rhizobial species (Table 1). Thus, continuous replanting of A. mearnsii in the same plot can intensify the selection of specific groups of rhizobia, consequently reducing diversity. A similar trend has been recorded for certain key leguminous cash crops, such as soybeans [113], cowpeas [114] and peanuts [115, 116], where the loss of rhizobial diversity led to a decline in plant vigour.
Why Is Crop Rotation Rarely Implemented in Plantation Forestry?
Even though crop rotation is beneficial to plant health, this is not a practice commonly implemented in short-rotation plantation forestry. There are multiple reasons for this. These include the fact that tree rotations are considerably longer (roughly 5 to 20 years for Eucalyptus and Pinus species under moderate climatic conditions) compared to the typical annual cycles of agronomic crops. Furthermore, compared to agriculture, fewer plant species are exploited in commercial forestry. The demand for specialised wood products and the availability of land that can be used for plantations are major challenges that discourage corporate and small forestry enterprises from implementing rotation programmes for plantation trees. Thus, populations of unfavourable microbes can be expected to become more abundant over successive rotations. This is strongly supported by data from recent soil microbiome studies involving commercially managed forests, which provide convincing evidence of an increase in pathogenic microbes in soils of continuously replanted forests [92••, 93••, 107•, 117]. Hence, despite the considerable challenges faced by commercial forestry, it is worth considering strategies to reduce or at least minimize the build-up of unfavourable microbes in planted forest environments.
Alternative Options to Mitigate the Negative Effect of Successive Replanting in Short-Rotation Forestry
In commercial forestry environments, two commonly used post-harvest residue management regimes are burning and mulching [118,119,120]. Burning is an economical and effective way to remove surplus residue, reduce fire hazards and manage pests and weeds [121,122,123]. It does, however, have a number of drawbacks, including the loss of soil nutrients, organic carbon and plant residues that reduce soil erosion [123, 124]. In contrast, retaining post-harvest residue and mulching with these residues can significantly enhance soil nutrient content, which is lost through continual replanting [125,126,127]. Retaining post-harvest residue also allows the restoration of soil microbes in continually replanted short-rotation plantations [126, 128, 129••]. Consequently, these microbes decompose the residues, allowing soil nutrients to be recycled as well as improve the physical and water retention properties of the soil [25, 122, 130].
A majority of studies assessing the efficacy of post-harvest residue management have focussed on quantifying the soil nutrients but rarely catalogue the community composition of microbes. However, Bose et al. [129••] recently evaluated the effects of three post-harvest residue management regimes, where residue was retained, removed, and removed and fertilized, on soil-associated fungal diversity in South Africa. This study showed that Eucalyptus plots where post-harvest residues were retained had a higher diversity of saprotrophs and symbiotrophs and fewer pathotrophs, compared to the other two regimes. In contrast, retention of tree stumps in plantations in the Northern Hemisphere increases the prevalence of Heterobasidion root rot among conifers. However, the removal of these stumps does not affect the biodiversity of beneficial microbes, such as mycorrhizae and saprotrophs [131, 132]. While these scenarios in Eucalyptus plantations and conifer forests are very different in nature, it highlights the potential that retained post-harvest residue could harbour certain pathogens. Therefore, further research is needed to verify the efficacy of various post-harvest residue management regimes in improving soil health and associated microbial biodiversity in relevant local scenarios.
Biochar is a carbon-rich, stable organic product made from the pyrolysis of organic biomasses such as leaves, sawdust, animal dung and wood [133]. During carbonization, biochar releases phosphate into the soil along with other mineral nutrients, improving its fertility [133,134]. Biochar also improves the physical properties [133] and microbial biodiversity of the soil, which could further increase soil nutrient availability and carbon storage [135,136,137]. However, the positive impact of biochar on soil is often contested [138,139]. In comparison to agriculture [140,141], our understanding of the impacts of biochar application on plantation forest soils is limited [133, 142•]. Some recent studies from commercial forestry settings have shown that biochar improved soil nutrients and microbes and reshapes the microbial community [142•, 143,144,145]. Early evidence is thus that biochar has considerable potential to enhance soil properties, nutrients and microbes in continuously replanted forests. Further research, however, is needed to acquire a better knowledge of its impacts on plantation soil health.
The use of beneficial microorganisms to improve plant health and sustainability is common in agriculture, but not in forestry [146,147,148]. This is due to the difficulty, low efficacy and cost of applying a microbial supplement to trees over large areas in forest environments. These treatments, however, can be potentially performed in nurseries at the seedling stage [149,150,151]. Mycorrhizal associations, for example, play an important role in a tree’s long-term survival in forests [9, 11]. However, the diversity and abundance of mycorrhizae and nitrogen-fixing bacteria are significantly lower in continually replanted forests [107•, 112••]. Treating the seedlings of commercial tree species in nurseries with mycorrhizae, nitrogen-fixing bacteria and endosymbiont mixtures could be explored as an option to promote planting success in commercial forests [149,150,151,152]. Diverse communities of these beneficial microbes could also allow planted seedlings to survive more readily in continuously replanted forest soil having a low nutrient content and a high concentration of harmful microbes [11, 153,154,155,156,157].
Adequate silviculture practices, such as crop rotation and intercropping, can alleviate the possible negative consequences of continuous replanting in short-rotation plantation forestry. Rotating between two distantly related tree species, such as Eucalyptus, Acacia mearnsii and conifers, can prevent the accumulation of harmful soil microbes detrimental to these trees (Fig. 2). For example, in South Africa, Eucalyptus and A. mearnsii are not infected by the same Phytophthora species. Eucalyptus species are susceptible to P. alticola, P. frigida and P. cinnamomi, whereas P. nicotianae infects A. mearnsii [158]. Thus, cycling between two non-host tree genera would likely reduce the population of either group of Phytophthora species to a level that will not cause a decline of either of the tree genera planted. Furthermore, rotating nitrogen-fixing leguminous tree species such as Acacia with mycorrhizal tree species like Eucalyptus or pines has the potential to further promote both soil and tree health (Fig. 2). Alternating between these trees could increase the availability of nitrogen in the soil through fixation [159] and improve the quality of plant litter [160], thereby enhancing the diversity of saprotrophic soil fungi [161], as well as improving mycorrhizal colonization [162]. Similarly, mycorrhizal fungi would also help to decompose leaf litter and mobilise essential nutrients in the soil, such as phosphorus [163, 164], and promote the sequestration of carbon [165].
Three potential schemes for minimizing the accumulation of detrimental microbes in monoculture plantations induced by the continuous replanting of the land with the same tree genus. Crop rotation involves alternating between mycorrhizal and rhizobial tree species such as Eucalyptus, Acacia and conifers. The other two systems involve intercropping with either two timber-producing trees or one each of timber and a crop plant such as legumes and tuber crops. When selecting the two timber schemes, intercropping a mycorrhizal and a rhizobial tree would be ideal. Intercropping of plantation trees and agricultural crops can allow agriculture and plantation to share land resources while also increasing soil carbon content and land productivity.
Future Research Needs and Opportunities
Most evidence for the build-up of soil-borne pathogens in planted forests emerges from short-term studies using short-read sequencing platforms. The diversity data from these studies provide some clues on the build-up of deleterious soil microbes due to continuous short to medium rotations of the same or nearly the same tree genotypes, yet they do not provide conclusive evidence [92••, 97, 98]. Even though short-read sequencing platforms are widely used to catalogue microbial diversity from various environments, they have several drawbacks, including limitations in taxonomic identification, taxonomic bias and amplification of dead microbes that are not a part of the immediate biodiversity, among others [94••, 166, 167]. Long-term monitoring programmes using third-generation (long-read) DNA sequencing platforms that improve taxonomic resolution and significantly reduce the possibility of amplifying dead organisms are required to address this shortfall in knowledge [168,169,170,171,172].
Long-term monitoring programs allow the sampling of soils from continuously replanted experimental plots at regular intervals. Microbiome data emerging from such experimental plots could be used to track the build-up of unfavourable microbes resulting from continuous replanting. Understanding the origin and perpetuation of disease-conducive soils in forestry environments will allow for the development and improvement of strategies to mitigate this problem. Simultaneously, species-level identification of these disease-conducive microbes is equally important for implementing mitigating strategies.
Although crop rotation is one of the most important strategies used in agriculture to mitigate the build-up of deleterious microbes, the efficacy of this approach has not been thoroughly tested in forestry environments, nor would it be practical for all forestry companies. An ideal approach to experimentally test the value of crop rotation would be to use relatively short rotations of Eucalyptus and Acacia as a model system (Fig. 2). This is because rotation and intercropping of these two tree species have been shown to improve the soil’s microbial diversity, nutrients and structure [162, 173, 174••, 175, 176]. Species of Pinus and other gymnosperms could also be included in these experimental rotations (Fig. 2).
In agroforestry, intercropping of timber-producing trees with agricultural crops such as legumes, tuber crops and a few others has also resulted in promising research outcomes [177,178,179,180] (Fig. 2). This approach allows sharing of land resources between agriculture and plantation forestry, while also enhancing soil carbon content and land productivity. Other advantages of this system include reduced soil erosion, weed management, improved biodiversity of soil microbes, improved soil quality, improved yield and yield stability and suppression of pests and pathogens [181,182]. Consequently, further research is needed to examine the feasibility of this system as a standard operational procedure. Soil microbiome data emerging from such studies at regular intervals would increase our understanding of the benefits of crop rotation and intercropping in managed forest environments. This could also result in environmentally and economically resilient plantations.
Advances in technologies are substantially influencing our understanding of the plant microbiome [183,184]. There is a clear shift in focus from issues relating to diversity towards a deeper understanding of changes in the functions of the microbial community in response to various environmental factors and their impact on tree health [185,186,187]. New techniques allow synthetic microbial communities (SynCom) to be designed with a defined set of microbes with known functions, such as improving plant immunity, nutrient acquisition and stress tolerance [188,189]. Such synthetic microbiomes make it possible to understand the effect of these communities on plant health in response to various environmental stresses, including plant pathogens [171, 183, 189,190,191]. For example, in maize, removing a single strain of Enterobacter cloacae disrupted a microbial community that was capable of lowering the severity of Fusarium verticillioides ear rot [192]. Similarly, in Arabidopsis thaliana, a synthetic microbiome has been utilised to predict plant phenotype [193]. However, the majority of these studies have focussed on microbes associated with crop plants or with model plants. Evaluating the influence of SynCom on the health of commercially important tree species, such as Eucalyptus, would be valuable.
In forest nurseries, fortifying plants with mycorrhizae and nitrifying bacteria can be explored to increase their establishment on constantly replanted land that is often low in biodiversity of beneficial microbes [149,150,151]. Nonetheless, the use of non-native but beneficial microbes could have unknown negative impacts, e.g. non-native strains aggressively competing with the native microbial population, which can impede the stability of the ecosystem [194,195,196,197,198,199,200]. Beneficial microbes should ideally be locally sourced strains that may be found in adjacent native forest patches and plantations because invasive plants frequently exploit them to colonise these environments [201,202,203,204,205,206]. Consequently, research is needed to identify these native beneficial microbes, such as ectomycorrhizae, develop strategies for their long-term establishment in plantations and assess their impact on the health and vigour of exotic plantation trees in non-native habitats.
Conclusions
Continuous replanting practised in short-rotation plantation forests is likely to be accompanied by a high risk of ‘replanting syndrome’ in plantations. While long-term monitoring programs to document the changes in soil microbiomes are still lacking and should be urgently initiated, the available evidence suggests that short-rotation forest plantation enterprises could be restrictive when successively establishing new plots with the same or nearly the same genotypes. Furthermore, it is necessary to assess the efficacy of crop rotation, inter-cropping, post-harvest residue management regimens and the inoculation of seedlings with beneficial microbes in treating this malady in short-rotation forestry environments.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Holmgren P (2021) The forest carbon debt illusion—contrary to common views, harvesting from managed forests does not delay climate benefits. Available online at: https://www.forestindustries.se/siteassets/dokument/rapporter/report-the-forest-carbon-debt-illusion2.pdf. Accessed 10 Oct 2022.
Food and Agriculture Organization. Global Forest Resources Assessment 2015. How are the world’s forests changing? Rome; 2016. https://www.fao.org/3/i4808e/i4808e.pdf. Accessed 15 Jan 2022.
Kirilenko AP, Sedjo RA. Climate change impacts on forestry. Proc Nat Acad Sci. 2007;104:19697–702.
McEwan A, Marchi E, Spinelli R, Brink M. Past, present and future of industrial plantation forestry and implication on future timber harvesting technology. J Forest Res. 2020;31:339–51.
Malkamäki A, D’Amato D, Hogarth NJ, Kanninen M, Pirard R, Toppinen A, Zhou W. A systematic review of the socio-economic impacts of large-scale tree plantations, worldwide. Global Environ Change. 2018;53:90–103.
McDonnell L, Coleman H, French D, Meilan R, Mansfield S. Engineering trees with target traits. Forests and Genetically Modified Trees: FAO, Rome; 2010. p. 77–122.
Häggman H, Raybould A, Borem A, Fox T, Handley L, Hertzberg M, Lu MZ, Macdonald P, Oguchi T, Pasquali G. Genetically engineered trees for plantation forests: key considerations for environmental risk assessment. Plant Biotechnol J. 2013;11:785–98.
Schulze J, Gawel E, Nolzen H, Weise H, Frank K. The expansion of short rotation forestry: characterization of determinants with an agent-based land use model. GCB Bioenergy. 2017;9:1042–56.
Baldrian P. Forest microbiome: diversity, complexity and dynamics. FEMS Microbiol Rev. 2017;41:109–30.
Raaijmakers JM, Paulitz TC, Steinberg C, Alabouvette C, Moënne-Loccoz Y. The rhizosphere: a playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant and Soil. 2009;321:341–61.
Berendsen RL, Pieterse CM, Bakker PA. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012;17:478–86.
Xia Z-C, Kong C-H, Chen L-C, Wang S-L. Allelochemical-mediated soil microbial community in long-term monospecific Chinese fir forest plantations. Appl Soil Ecol. 2015;96:52–9.
•• Wu Z, Li J, Zheng J, Liu J, Liu S, Lin W, Wu C. Soil microbial community structure and catabolic activity are significantly degenerated in successive rotations of Chinese fir plantations. Sci Rep. 2017;7:6691 This study showed that continuous replanting of Chinese fir on the same land leads to a decline in catabolic activity and the community diversity of soil microbes.
• Bini D, dos Santos CA, do Carmo KB, Kishino N, Andrade G, Zangaro W, Nogueira MA. Effects of land use on soil organic carbon and microbial processes associated with soil health in southern Brazil. Eur J Soil Biol. 2013;55:117–23 The study assessed soil health in relation to carbon cycling in soils under two land uses, mixed ombrophilous forest versus non-native monoclonal plantations, and developed markers that may be used to track the effects of land-use changes.
Li Y, Xu M, Zou X, Xia Y. Soil CO 2 efflux and fungal and bacterial biomass in a plantation and a secondary forest in wet tropics in Puerto Rico. Plant Soil. 2005;268:151–60.
Park C-W, Ko S, Yoon TK, Han S, Yi K, Jo W, Jin L, Lee SJ, Noh NJ, Chung H. Differences in soil aggregate, microbial biomass carbon concentration, and soil carbon between Pinus rigida and Larix kaempferi plantations in Yangpyeong, central Korea. Forest Sci Technol. 2012;8:38–46.
Wiesmeier M, Dick D, Rumpel C, Dalmolin R, Hilscher A, Knicker H. Depletion of soil organic carbon and nitrogen under Pinus taeda plantations in Southern Brazilian grasslands (Campos). Eu J Soil Sci. 2009;60:347–59.
He J, Xu Z, Hughes J. Analyses of soil fungal communities in adjacent natural forest and hoop pine plantation ecosystems of subtropical Australia using molecular approaches based on 18S rRNA genes. FEMS Microbiol Lett. 2005;247:91–100.
Pan K, Xu Z, Blumfield TJ, Tutua S, Lu M. Application of (15NH4)2 SO4 to study N dynamics in hoop pine plantation and adjacent native forest of subtropical Australia: the effects of injection depth and litter addition. J Soils Sed. 2009;9:515–25.
He J-Z, Ge Y, Xu Z, Chen C. Linking soil bacterial diversity to ecosystem multifunctionality using backward-elimination boosted trees analysis. J Soils Sed. 2009;9:547.
Xu Z, Chen C, He J, Liu J. Trends and challenges in soil research 2009: linking global climate change to local long-term forest productivity. J Soil Sed. 2009;9:83–8.
Toor MD, Adnan M. Role of soil microbes in agriculture: a review. J Biogen Res. 2020;10:1–5.
Jing X, Chen X, Fang J, Ji C, Shen H, Zheng C, Zhu B. Soil microbial carbon and nutrient constraints are driven more by climate and soil physicochemical properties than by nutrient addition in forest ecosystems. Soil Biol Biochem. 2020;141:107657.
Breland TA, Hansen S. Nitrogen mineralization and microbial biomass as affected by soil compaction. Soil Biol Biochem. 1996;28:655–63.
Li Q, Allen HL, Wollum AG II. Microbial biomass and bacterial functional diversity in forest soils: effects of organic matter removal, compaction, and vegetation control. Soil Biol Biochem. 2004;36:571–9.
Luizao RC, Bonde TA, Rosswall T. Seasonal variation of soil microbial biomass-the effects of clearfelling a tropical rainforest and establishment of pasture in the Central Amazon. Soil Biol Biochem. 1992;24:805–13.
Pietikäinen J, Fritze H. Clear-cutting and prescribed burning in coniferous forest: comparison of effects on soil fungal and total microbial biomass, respiration activity and nitrification. Soil Biol Biochem. 1995;27:101–9.
Dubey A, Malla MA, Khan F, Chowdhary K, Yadav S, Kumar A, Sharma S, Khare PK, Khan ML. Soil microbiome: a key player for conservation of soil health under changing climate. Biodivers Conserv. 2019;28:2405–29.
Trentini CP, Campanello PI, Villagra M, Ferreras J, Hartmann M. Thinning partially mitigates the impact of Atlantic forest replacement by pine monocultures on the soil microbiome. Front Microbiol. 2020;11:1491.
•• Guo J, Feng H, McNie P, Wang W, Peng C, Feng L, Sun J, Pan C, Yu Y. The effect of the conversion from natural broadleaved forests into Chinese fir (Cunninghamia lanceolata (lamb.) Hook.) plantations on soil microbial communities and nitrogen functional genes. Forests. 2022;13:158 The article investigates the detrimental effects of changing natural forests with broadleaf tree species to monoclonal Chinese fir on soil properties and microbial communities.
Cheng Y, Zhou L, Liang T, Man J, Wang Y, Li Y, Chen H, Zhang T. Deciphering rhizosphere microbiome assembly of Castanea henryi in plantation and natural forest. Microorganisms. 2022;10:42.
Curlevski NJA, Xu Z, Anderson IC, Cairney JWG. Soil fungal communities differ in native mixed forest and adjacent Araucaria cunninghamii plantations in subtropical Australia. J Soils Sed. 2010;10:1278–88.
Jiang Y, Chen C, Xu Z, Liu Y. Effects of single and mixed species forest ecosystems on diversity and function of soil microbial community in subtropical China. J Soils Sed. 2012;12:228–40.
Bing-Cheng Y, Dong-Xia Y. Soil microbial and enzymatic activities across a chronosequence of Chinese pine plantation development on the Loess plateau of China. Pedosphere. 2012;22:1–12.
van der Wal A, van Veen JA, Smant W, Boschker HTS, Bloem J, Kardol P, van der Putten WH, de Boer W. Fungal biomass development in a chronosequence of land abandonment. Soil Biol Biochem. 2006;38:51–60.
Lauber CL, Strickland MS, Bradford MA, Fierer N. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol Biochem. 2008;40:2407–15.
Oria-de-Rueda JA, Hernández-Rodríguez M, Martín-Pinto P, Pando V, Olaizola J. Could artificial reforestations provide as much production and diversity of fungal species as natural forest stands in marginal Mediterranean areas? Forest Ecol Manag. 2010;260:171–80.
Smith SE, Read DJ. Mycorrhizal symbiosis: Academic Press; 2010.
Garbaye J. Helper bacteria: a new dimension to the mycorrhizal symbiosis. New Phytol. 1994;128:197–210.
Mallik MAB, Williams RD. Plant growth promoting rhizobacteria and mycorrhizal fungi in sustainable agriculture and forestry. In: Zeng RS, Mallik AU, Luo SM, editors. Allelopathy in sustainable agriculture and forestry. New York, NY: Springer New York; 2008. p. 321–45.
Rincón A, Ruíz-Díez B, Fernández-Pascual M, Probanza A, Pozuelo JM, De Felipe M. Afforestation of degraded soils with Pinus halepensis Mill.: effects of inoculation with selected microorganisms and soil amendment on plant growth, rhizospheric microbial activity and ectomycorrhizal formation. Appl Soil Ecol. 2006;34:42–51.
Freeman J, Ward E. Gaeumannomyces graminis, the take-all fungus and its relatives. Mol Plant Pathol. 2004;5:235–52.
Hornby D. Take-all disease of cereals: a regional perspective: CABI International; 1998.
Palma-Guerrero J, Chancellor T, Spong J, Canning G, Hammond J, McMillan VE, Hammond-Kosack KE. Take-all disease: new insights into an important wheat root pathogen. Trends Plant Sci. 2021;26:836–48.
Bockus W, Tisserat N. Take-all root rot. The Plant Health Instructor. 2000. https://www.apsnetorg/edcenter/disandpath/fungalasco/pdlessons/Pages/Takeallaspx. Accessed 22 July 2022.
Liu C, Shang H, Tan R. Infection process of take-all causing fungus (Gaeumannomyces graminis var. tritici) on wheat (Triticum aestivum) and oat (Avena sativa) roots. Ind J Agricult Sci. 2000;70:23–7.
• Cook RJ. Untold stories: forty years of field research on root diseases of wheat: The American Phytopathological Society; 2017. This book summarises both historic and recent advances in take-all disease research.
Cook RJ. The influence of rotation crops on take-all decline phenomenon. Phytopathology. 1981;71:189–92.
Braun PG, Fuller KD, McRae K, Fillmore SAE. Response of ‘Honeycrisp®’apple trees to combinations of pre-plant fumigation, deep ripping, and hog manure compost incorporation in a soil with replant disease. HortScience. 2010;45:1702–7.
• Mazzola M. Elucidation of the microbial complex having a causal role in the development of apple replant disease in Washington. Phytopathology. 1998;88:930–8 This study investigates the role of various soil-borne microbes in the development of apple replant disease. The outcome of this study showed that fungi are the principle causal agents of apple replant disease in Washington state.
van Schoor L, Denman S, Cook NC. Characterisation of apple replant disease under South African conditions and potential biological management strategies. Sci Horticult. 2009;119:153–62.
Isutsa DK, Merwin IA. Malus germplasm varies in resistance or tolerance to apple replant disease in a mixture of New York orchard soils. HortScience. 2000;35:262–8.
Yim B, Smalla K, Winkelmann T. Evaluation of apple replant problems based on different soil disinfection treatments—links to soil microbial community structure? Plant Soil. 2013;366:617–31.
Caruso FL, Neubauer BF, Begin MD. A histological study of apple roots affected by replant disease. Canad J Botany. 1989;67:742–9.
Mazzola M, Manici LM. Apple replant disease: role of microbial ecology in cause and control. Ann Rev Phytopathol. 2012;50:45–65.
Chen T, Lin S, Wu LK, Lin WX, Sampietro DA. Soil sickness: current status and future perspectives. Allelopathy J. 2015;36:167–96.
Winkelmann T, Smalla K, Amelung W, Baab G, Grunewaldt-Stöcker G, Kanfra X, Meyhöfer R, Reim S, Schmitz M, Vetterlein D. Apple replant disease: causes and mitigation strategies. Cur Issues Mol Biol. 2018;30:89–106.
Mazzola M, Gu Y-H. Impact of wheat cultivation on microbial communities from replant soils and apple growth in greenhouse trials. Phytopathology. 2000;90:114–9.
Redman RS, Dunigan DD, Rodriguez RJ. Fungal symbiosis from mutualism to parasitism: who controls the outcome, host or invader? New Phytol. 2001;151:705–16.
Kelderer M, Manici LM, Caputo F, Thalheimer M. Planting in the ‘inter-row’to overcome replant disease in apple orchards: a study on the effectiveness of the practice based on microbial indicators. Plant Soil. 2012;357:381–93.
Manici L, Kelderer M, Franke-Whittle IH, Rühmer T, Baab G, Nicoletti F, Caputo F, Topp A, Insam H, Naef A. Relationship between root-endophytic microbial communities and replant disease in specialized apple growing areas in Europe. Appl Soil Ecol. 2013;72:207–14.
Tewoldemedhin YT, Mazzola M, Botha WJ, Spies CFJ, McLeod A. Characterization of fungi (Fusarium and Rhizoctonia) and oomycetes (Phytophthora and Pythium) associated with apple orchards in South Africa. Eur J Plant Pathol. 2011;130:215–29.
Tewoldemedhin YT, Mazzola M, Labuschagne I, McLeod A. A multi-phasic approach reveals that apple replant disease is caused by multiple biological agents, with some agents acting synergistically. Soil Biol Biochem. 2011;43:1917–27.
Peruzzi E, Franke-Whittle IH, Kelderer M, Ciavatta C, Insam H. Microbial indication of soil health in apple orchards affected by replant disease. Appl Soil Ecol. 2017;119:115–27.
Nicola L, Insam H, Pertot I, Stres B. Reanalysis of microbiomes in soils affected by apple replant disease (ARD): old foes and novel suspects lead to the proposal of extended model of disease development. Appl Soil Ecol. 2018;129:24–33.
Yin C, Xiang L, Wang G, Wang Y, Shen X, Chen X, Mao Z. How to plant apple trees to reduce replant disease in apple orchard: a study on the phenolic acid of the replanted apple orchard. PLoS One. 2016;11:e0167347.
Li C, Zhao Q, Gao T, Wang H, Zhang Z, Liang B, Wei Z, Liu C, Ma F. The mitigation effects of exogenous melatonin on replant disease in apple. J Pineal Res. 2018;65:e12523.
Freeman J, Ward E, Gutteridge RJ, Bateman GL. Methods for studying population structure, including sensitivity to the fungicide silthiofam, of the cereal take-all fungus Gaeumannomyces graminis var. tritici. Plant Pathol. 2005;54:686–98.
Zhu Y, Fazio G, Mazzola M. Elucidating the molecular responses of apple rootstock resistant to ARD pathogens: challenges and opportunities for development of genomics-assisted breeding tools. Horticult Res. 2014;1:14043.
Yates F. The analysis of experiments containing different crop rotations. Biometrics. 1954;10:324–46.
Stinner BR, Blair JM. Ecological and agronomic characteristics of innovative cropping systems. In: Edwards CA, Lal R, Madden P, Miller RH, House G, editors. Sustainable Agricultural Systems. Soil and Water Conservation Society: Ankeny USA; 1990. p. 123–40.
Blakney AJC, Bainard LD, St-Arnaud M, Hijri M. Soil chemistry and soil history significantly structure oomycete communities in Brassicaceae crop rotations. Appl Environ Microbiol. 2023;89:e0131422.
Hornby D. Suppressive soils. Ann Rev Phytopathol. 1983;21:65–85.
Bullock DG. Crop rotation. Crit Rev Plant Sci. 1992;11:309–26.
Tilman D, Cassman KG, Matson PA, Naylor R, Polasky S. Agricultural sustainability and intensive production practices. Nature. 2002;418:671–7.
Castellazzi M, Wood G, Burgess PJ, Morris J, Conrad K, Perry J. A systematic representation of crop rotations. Agricult Syst. 2008;97:26–33.
Boyabatlı O, Nasiry J, Zhou Y. Crop planning in sustainable agriculture: dynamic farmland allocation in the presence of crop rotation benefits. Manag Sci. 2019;65:2060–76.
Bailey K, Duczek L. Managing cereal diseases under reduced tillage. Canad J Plant Pathol. 1996;18:159–67.
Huisman O, Ashworth L Jr. Influence of crop rotation on survival of Verticillium albo-atrum in soils. Phytopathology. 1976;66:978–81.
Umaerus V, Scholte K, Turkensteen L. Crop rotation and the occurrence of fungal diseases in potatoes. In: Effects of crop rotation on potato production in the temperate zones: Springer; 1989. p. 171–89.
Tillmann M, von Tiedemann A, Winter M. Crop rotation effects on incidence and diversity of Fusarium species colonizing stem bases and grains of winter wheat. J Plant Dis Protect. 2017;124:121–30.
Neupane A, Bulbul I, Wang Z, Lehman RM, Nafziger E, Marzano S-YL. Long term crop rotation effect on subsequent soybean yield explained by soil and root-associated microbiomes and soil health indicators. Sci Rep. 2021;11:1–13.
Edwards J, Thurlow D, Eason J. Influence of tillage and crop rotation on yields of corn, soybean, and wheat. Agronomy J. 1988;80:76–80.
Lupwayi NZ, Fernandez MR, Kanashiro DA, Petri RM. Profiles of wheat rhizobacterial communities in response to repeated glyphosate applications, crop rotation, and tillage. Canad J Soil Sci. 2020;101:157–67.
Kumar R, Mishra JS, Rao KK, Mondal S, Hazra KK, Choudhary JS, Hans H, Bhatt BP. Crop rotation and tillage management options for sustainable intensification of rice-fallow agro-ecosystem in eastern India. Sci Rep. 2020;10:11146.
Xuan DT, Guong VT, Rosling A, Alström S, Chai B, Högberg N. Different crop rotation systems as drivers of change in soil bacterial community structure and yield of rice, Oryza sativa. Biol Fertil Soils. 2012;48:217–25.
Breidenbach B, Blaser MB, Klose M, Conrad R. Crop rotation of flooded rice with upland maize impacts the resident and active methanogenic microbial community. Environ Microbiol. 2016;18:2868–85.
Specht L, Leach S. Effects of crop rotation on Rhizoctonia disease of white potato. Plant Dis. 1987;71:433–7.
Frank JA, Murphy H. The effect of crop rotations on Rhizoctonia disease of potatoes. Am Potato J. 1977;54:315–22.
Johnston H, Celetti M, Kimpinski J, Platt H. Fungal pathogens and Pratylenchus penetrans associated with preceding crops of clovers, winter wheat, and annual ryegrass and their influence on succeeding potato crops on Prince Edward Island. Am Potato J. 1994;71:797–808.
Peters RD, Sturz AV, Carter MR, Sanderson JB. Influence of crop rotation and conservation tillage practices on the severity of soil-borne potato diseases in temperate humid agriculture. Canad J Soil Sci. 2004;84:397–402.
•• Jimu L, Kemler M, Mujuru L, Mwenje E. Illumina DNA metabarcoding of Eucalyptus plantation soil reveals the presence of mycorrhizal and pathogenic fungi. Forestry. 2018;91:238–45 This study compared the community composition of soil fungi associated with exotic Eucalyptus grandis and adjacent woodlands in Zimbabwe. The soil from the plantation included fungi that are Eucalyptus pathogens.
•• Byers A-K, Condron L, Donavan T, O'Callaghan M, Patuawa T, Waipara N, Black A. Soil microbial diversity in adjacent forest systems—contrasting native, old growth kauri (Agathis australis) forest with exotic pine (Pinus radiata) plantation forest. FEMS Microbiol Ecol. 96:2020, iaa047 This study compared the fungal and bacterial biodiversity between plantations of exotic Pinus radiata and adjacent stands of old-growth Kauri forest. The plantation soils had poor soil properties and were pathogen reservoirs that could, in the future, jeopardise the sustainability of the Kauri forest.
•• Bose T, Wingfield MJ, Roux J, Vivas M, Burgess TI. Community composition and distribution of Phytophthora species across adjacent native and non-native forests of South Africa. Fungal Ecol. 2018;36:17–25 This study compared the community composition and richness of Phytophthora species between plantations of exotic tree species and adjacent natural forest patches. The plantation soils included Phytophthora species that are pathogenic to those trees.
•• Wu Z, Haack SE, Lin W, Li B, Wu L, Fang C, Zhang Z. Soil microbial community structure and metabolic activity of Pinus elliottii plantations across different stand ages in a subtropical area. PLoS One. 2015;10:e0135354 This study reported that long-term monoculture of Pinus elliottii significantly reduced soil microbial community diversity and metabolic activity.
Zhang Z, Zhong Y, Yang L, Li D, Tang H, He J. Landscape pattern and succession of Chinese fir plantations in Jiangle County China. Sustainability. 2022;14:12497.
Riddell CE, Frederickson-Matika D, Armstrong AC, Elliot M, Forster J, Hedley PE, Morris J, Thorpe P, Cooke DE, Pritchard L, Sharp PM, Green S. Metabarcoding reveals a high diversity of woody host-associated Phytophthora spp. in soils at public gardens and amenity woodlands in Britain. PeerJ. 2019;7:e6931.
Bose T, Wingfield MJ, Roux J, Vivas M, Burgess TI. Phytophthora species associated with roots of native and non-native trees in natural and managed forests. Microbial Ecol. 2020;81:122–133.
Shi L, Dossa GGO, Paudel E, Zang H, Xu J, Harrison RD. Changes in fungal communities across a forest disturbance gradient. Appl Environ Microbiol. 2019;85:e00080–19.
Zhao M, Zhao J, Yuan J, Hale L, Wen T, Huang Q, Vivanco JM, Zhou J, Kowalchuk GA, Shen Q. Root exudates drive soil-microbe-nutrient feedbacks in response to plant growth. Plant, Cell Environ. 2021;44:613–28.
Etalo DW, Jeon J-S, Raaijmakers JM. Modulation of plant chemistry by beneficial root microbiota. Nat Prod Rep. 2018;35:398–409.
Voříšková J, Baldrian P. Fungal community on decomposing leaf litter undergoes rapid successional changes. ISME J. 2013;7:477–86.
Boddy L. Interspecific combative interactions between wood-decaying Basidiomycetes. FEMS Microbio Ecol. 2000;31:185–94.
Crowther TW, Boddy L, Jones T, H. Functional and ecological consequences of saprotrophic fungus–grazer interactions. The ISME Journal. 2012;6:1992–2001.
•• Xu Y, Li C, Zhu Y, Wang Z, Zhu W, Wu L, Du A. The shifts in soil microbial community and association network induced by successive planting of Eucalyptus plantations. Forest Ecol Manag. 2022;505:119877 This study conducted long-term research to determine the influence of continous planting of Eucalyptus on soil. Data from this study showed continous cropping of Eucalyptus on same land had a detrimental influence on soil multifunctionality and microbial communities.
• Xu Y, Ren S, Liang Y, Du A, Li C, Wang Z, Zhu W, Wu L. Soil nutrient supply and tree species drive changes in soil microbial communities during the transformation of a multi-generation Eucalyptus plantation. Appl Soil Ecol. 2021;166:103991 This study compared soil health and microbial biodiversity between monoclonal Eucalyptus plots and those interplanted with Manglietia glauca. Interplanting plots with tree species enhanced soil fertility and increased the number and richness of beneficial fungi and bacteria.
• Chen YL, Liu S, Dell B. Mycorrhizal status of Eucalyptus plantations in south China and implications for management. Mycorrhiza. 2007;17:527–35 This study revealed that plots that are continually replanted with Eucalyptus have reduced biodiversity of ecto and endo mycorrhizae.
Castano C, Dejene T, Mediavilla O, Geml J, Oria-de-Rueda JA, Martín-Pinto P. Changes in fungal diversity and composition along a chronosequence of Eucalyptus grandis plantations in Ethiopia. Fungal Ecol. 2019;39:328–35.
Zheng Y, Hu H-W, Guo L-D, Anderson IC, Powell JR. Dryland forest management alters fungal community composition and decouples assembly of root- and soil-associated fungal communities. Soil Biol Biochem. 2017;109:14–22.
Scott PM, Shearer BL, Barber PA, Hardy GESJ. Relationships between the crown health, fine root and ectomycorrhizae density of declining Eucalyptus gomphocephala. Aust Plant Pathol. 2013;42:121–31.
Ishaq L, Barber PA, Hardy GESJ, Calver M, Dell B. Seedling mycorrhizal type and soil chemistry are related to canopy condition of Eucalyptus gomphocephala. Mycorrhiza. 2013;23:359–71.
•• de São José JFB, Hernandes MAS, Volpiano CG, Lisboa BB, Beneduzi A, Bayer C, Simon AA, de Oliveira J, Passaglia LMP, Vargas LK. Diversity of rhizobia, symbiotic effectiveness, and potential of inoculation in Acacia mearnsii seedling production. Br J Microbiol. 2023;54:335–48 This study compared the rhizobial diversity associated with A. mearnsii. The genetic diversity of rhizobial species was higher in newly planted A. mearnsii plots, whereas it was low in continuously replanted plots.
Coutinho HL, Oliveira VM, Lovato A, Maia AH, Manfio GP. Evaluation of the diversity of rhizobia in Brazilian agricultural soils cultivated with soybeans. Appl Soil Ecol. 1999;13:159–67.
Zilli JÉ, Valisheski RR, Freire Filho FR, Neves MCP, Rumjanek NG. Assessment of cowpea rhizobium diversity in Cerrado areas of northeastern Brazil. Br J Microbiol. 2004;35:281–7.
Nkot LN, Krasova-Wade T, Etoa F, Sylla S, Nwaga D. Genetic diversity of rhizobia nodulating Arachis hypogaea L. in diverse land use systems of humid forest zone in Cameroon. Appl Soil Ecol. 2008;40:411–6.
Shao S, Chen M, Liu W, Hu X, Wang E-T, Yu S, Li Y. Long-term monoculture reduces the symbiotic rhizobial biodiversity of peanut. Syst Appl Microbiol. 2020;43:126101.
Gazol A, Zobel M, Cantero JJ, Davison J, Esler KJ, Jairus T, Öpik M, Vasar M, Moora M. Impact of alien pines on local arbuscular mycorrhizal fungal communities-evidence from two continents. FEMS Microbiol Ecol. 2016;92:fiw073.
Gonçalves JLM, Wichert MCP, Gava JL, Masetto AV, Junior AJC, Serrano MIP, Mello SLM. Soil fertility and growth of Eucalyptus grandis in Brazil under different residue management practices. Southern Hemis Forestry J. 2007;69:95–102.
Rocha JHT, Gonçalves JLM, Gava JL, Godinho TO, Melo EASC, Bazani JH, Hubner A, Arthur Junior JC, Wichert MP. Forest residue maintenance increased the wood productivity of a Eucalyptus plantation over two short rotations. Forest Ecol Manag. 2016;379:1–10.
Pellegrini AFA, Harden J, Georgiou K, Hemes KS, Malhotra A, Nolan CJ, Jackson RB. Fire effects on the persistence of soil organic matter and long-term carbon storage. Nat Geosci. 2022;15:5–13.
Jang W, Page-Dumroese DS, Han H-S. Comparison of heat transfer and soil impacts of air curtain burner burning and slash pile burning. Forests. 2017;8:297.
Mendham DS, O’connell AM, Grove TS, Rance SJ. Residue management effects on soil carbon and nutrient contents and growth of second rotation eucalypts. Forest Ecol Manag. 2003;181:357–72.
Rhoades CC, Fornwalt PJ. Pile burning creates a fifty-year legacy of openings in regenerating lodgepole pine forests in Colorado. Forest Ecol Manag. 2015;336:203–9.
Certini G. Effects of fire on properties of forest soils: a review. Oecologia. 2005;143:1–10.
de Moraes Goncalves JL, Alvares CA, Higa AR, Silva LD, Alfenas AC, Stahl J, de Barros Ferraz SF, de Paula LW, Brancalion PHS, Hubner A. Integrating genetic and silvicultural strategies to minimize abiotic and biotic constraints in Brazilian eucalypt plantations. Forest Ecol Manag. 2013;301:6–27.
Mendham DS, Sankaran KV, O'Connell AM, Grove TS. Eucalyptus globulus harvest residue management effects on soil carbon and microbial biomass at 1 and 5 years after plantation establishment. Soil Biol Biochem. 2002;34:1903–12.
Kumaraswamy S, Mendham DS, Grove TS, O’Connell AM, Sankaran KV, Rance SJ. Harvest residue effects on soil organic matter, nutrients and microbial biomass in eucalypt plantations in Kerala India. Forest Ecol Manag. 2014;328:140–9.
He J, Xu Z, Hughes J. Molecular bacterial diversity of a forest soil under residue management regimes in subtropical Australia. FEMS Microbiol Ecol. 2006;55:38–47.
•• Bose T, Vivas M, Slippers B, Roux J, Kemler M, Begerow D, Witfeld F, Brachmann A, Dovey S, Wingfield MJ. Retention of post-harvest residues enhances soil fungal biodiversity in Eucalyptus plantations. Forest Ecol Manag. 2023;532:120806 This study compared the effect of three post-harvest residue management regimes on biodiversity of soil fungi in Eucalyptus plantations. The plots retaining post-harvest residue have a higher biodiversity of saprotrophs and symbiotrophs and fewer pathotrophs.
Smith JL, Paul EA. The significance of soil microbial biomass estimations. In: Bollag JM, Stotzky G, editors. Soil Biochemistry, vol. 6. Marcel Dekker: New York; 1990. p. 357–96.
Burnevica N, Klavina D, Polmanis K, Jansons J, Gaitnieks T. Impact of stump removal on communities of ectomycorrhizal and other soil fungi in Norway spruce stands of Latvia. Diversity. 2022;14:673.
Burņeviča N, Zaļuma A, Kļaviņa D, Brūna L, Legzdiņa L, Gaitnieks T. Initial and long-term fungal diversity and occurrence of Heterobasidion spp. in Norway spruce root fragments remaining in soil after stump extraction. Scand J Forest Res. 2021;36:117–25.
Li Y, Hu S, Chen J, Müller K, Li Y, Fu W, Lin Z, Wang H. Effects of biochar application in forest ecosystems on soil properties and greenhouse gas emissions: a review. J Soils Sed. 2018;18:546–63.
de la Rosa JM, Paneque M, Miller AZ, Knicker H. Relating physical and chemical properties of four different biochars and their application rate to biomass production of Lolium perenne on a Calcic Cambisol during a pot experiment of 79 days. Sci Total Environ. 2014;499:175–84.
Anderson CR, Condron LM, Clough TJ, Fiers M, Stewart A, Hill RA, Sherlock RR. Biochar induced soil microbial community change: implications for biogeochemical cycling of carbon, nitrogen and phosphorus. Pedobiologia. 2011;54:309–20.
Mitchell PJ, Simpson AJ, Soong R, Schurman JS, Thomas SC, Simpson MJ. Biochar amendment and phosphorus fertilization altered forest soil microbial community and native soil organic matter molecular composition. Biogeochemistry. 2016;130:227–45.
Palansooriya KN, Wong JTF, Hashimoto Y, Huang L, Rinklebe J, Chang SX, Bolan N, Wang H, Ok YS. Response of microbial communities to biochar-amended soils: a critical review. Biochar. 2019;1:3–22.
Liu S, Zhang Y, Zong Y, Hu Z, Wu S, Zhou J, Jin Y, Zou J. Response of soil carbon dioxide fluxes, soil organic carbon and microbial biomass carbon to biochar amendment: a meta-analysis. GCB Bioenergy. 2016;8:392–406.
Noyce GL, Winsborough C, Fulthorpe R, Basiliko N. The microbiomes and metagenomes of forest biochars. Sci Rep. 2016;6:26425.
Atkinson CJ, Fitzgerald JD, Hipps NA. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: a review. Plant Soil. 2010;337:1–18.
El-Naggar A, Lee SS, Rinklebe J, Farooq M, Song H, Sarmah AK, Zimmerman AR, Ahmad M, Shaheen SM, Ok YS. Biochar application to low fertility soils: a review of current status, and future prospects. Geoderma. 2019;337:536–54.
• Zhou C, Heal K, Tigabu M, Xia L, Hu H, Yin D, Ma X. Biochar addition to forest plantation soil enhances phosphorus availability and soil bacterial community diversity. Forest Ecol Manag. 2020;455:117635 This study demonstrated that adding biochar to the soil is a viable alternative for increasing P availability and helping to save or minimise nutrient losses for the following cycle.
Cui J, Glatzel S, Bruckman VJ, Wang B, Lai DYF. Long-term effects of biochar application on greenhouse gas production and microbial community in temperate forest soils under increasing temperature. Sci Total Environ. 2021;767:145021.
Lasota J, Błońska E, Babiak T, Piaszczyk W, Stępniewska H, Jankowiak R, Boroń P, Lenart-Boroń A. Effect of charcoal on the properties, enzyme activities and microbial diversity of temperate pine forest soils. Forests. 2021;12:1488.
Wang M, Yu X, Weng X, Zeng X, Li M, Sui X. Meta-analysis of the effects of biochar application on the diversity of soil bacteria and fungi. Microorganisms. 2023;11:641.
Santos MS, Nogueira MA, Hungria M. Microbial inoculants: reviewing the past, discussing the present and previewing an outstanding future for the use of beneficial bacteria in agriculture. AMB Express. 2019;9:205.
Verma P, Yadav AN, Kumar V, Singh DP, Saxena AK. Beneficial plant-microbes interactions: biodiversity of microbes from diverse extreme environments and Its Impact for crop improvement. In: Singh DP, Singh HB, Prabha R, editors. Plant-microbe interactions in agro-ecological perspectives: volume 2: microbial interactions and agro-ecological impacts. Springer: Singapore; 2017. p. 543–80.
Suman A, Yadav AN, Verma P. Endophytic microbes in crops: diversity and beneficial impact for sustainable agriculture. In: Singh DP, Singh HB, Prabha R, editors. Microbial Inoculants in Sustainable Agricultural Productivity, vol. 1. New Delhi: Research Perspectives. Springer India; 2016. p. 117–43.
Munro RC, Wilson J, Jefwa J, Mbuthia KW. A low-cost method of mycorrhizal inoculation improves growth of Acacia tortilis seedlings in the nursery. Forest Ecol Manag. 1999;113:51–6.
Ortega U, Duñabeitia M, Menendez S, Gonzalez-Murua C, Majada J. Effectiveness of mycorrhizal inoculation in the nursery on growth and water relations of Pinus radiata in different water regimes. Tree Physiol. 2004;24:65–73.
Urgiles N, Strauß A, Loján P, Schüßler A. Cultured arbuscular mycorrhizal fungi and native soil inocula improve seedling development of two pioneer trees in the Andean region. New Forests. 2014;45:859–74.
Zhang Z, Mallik A, Zhang J, Huang Y, Zhou L. Effects of arbuscular mycorrhizal fungi on inoculated seedling growth and rhizosphere soil aggregates. Soil Tillage Res. 2019;194:104340.
Naidoo S, Slippers B, Plett JM, Coles D, Oates CN. The road to resistance in forest trees. Front Plant Sci. 2019;10:273.
Sapsford SJ, Paap T, Hardy GESJ, Burgess TI. The ‘chicken or the egg’: which comes first, forest tree decline or loss of mycorrhizae? Plant Ecol. 2017;218:1093–106.
Rabiey M, Hailey LE, Roy SR, Grenz K, Al-Zadjali MA, Barrett GA, Jackson RW. Endophytes vs tree pathogens and pests: can they be used as biological control agents to improve tree health? Eu J Plant Pathol. 2019;155:711–29.
Puri A, Padda KP, Chanway CP. Can naturally-occurring endophytic nitrogen-fixing bacteria of hybrid white spruce sustain boreal forest tree growth on extremely nutrient-poor soils? Soil Biol Biochem. 2020;140:107642.
Videira e Castro I, de Castro Silva M, Fernandez C, Colavolpe B, Machado H. The potential of nitrogen-fixing bacteria in the sustainability of agro-forestry ecosystems. In: Zúñiga-Dávila D, González-Andrés F, Ormeño-Orrillo E, editors. Microbial probiotics for agricultural systems: advances in agronomic use. Cham: Springer International Publishing; 2019. p. 71–82.
Bose T, Roux J, Burgess TI, Shaw C, Wingfield MJ. Susceptibility of Eucalyptus grandis and Acacia mearnsii seedlings to five Phytophthora species common in South African plantations. Forest Pathol. 2019;49:e12560.
Bouillet J-P, Laclau J-P, Gonçalves JM, Moreira MZ, Trivelin PCO, Jourdan C, Silva E, Piccolo MC, Tsai SM, Galiana A. Mixed-species plantations of Acacia mangium and Eucalyptus grandis in Brazil: 2: nitrogen accumulation in the stands and biological N2 fixation. Forest Ecol Manag. 2008;255:3918–30.
Forrester DI, Bauhus J, Cowie AL. Nutrient cycling in a mixed-species plantation of Eucalyptus globulus and Acacia mearnsii. Canad J Forest Res. 2005;35:2942–50.
Li Y, Bezemer TM, Yang J, Lü X, Li X, Liang W, Han X, Li Q. Changes in litter quality induced by N deposition alter soil microbial communities. Soil Biol Biochem. 2019;130:33–42.
Bini D, Santos CA, Silva MCP, Bonfim JA, Cardoso EJBN. Intercropping Acacia mangium stimulates AMF colonization and soil phosphatase activity in Eucalyptus grandis. Sci Agr. 2018;75:102–10.
Tedersoo L, Bahram M. Mycorrhizal types differ in ecophysiology and alter plant nutrition and soil processes. Biol Rev. 2019;94:1857–80.
Johnson NC, Wilson GW, Wilson JA, Miller RM, Bowker MA. Mycorrhizal phenotypes and the law of the minimum. New Phytol. 2015;205:1473–84.
Forrester DI, Bauhus J, Cowie AL. Carbon allocation in a mixed-species plantation of Eucalyptus globulus and Acacia mearnsii. Forest Ecol Manag. 2006;233:275–84.
Nilsson RH, Anslan S, Bahram M, Wurzbacher C, Baldrian P, Tedersoo L. Mycobiome diversity: high-throughput sequencing and identification of fungi. Nat Rev Microbiol. 2019;17:95–109.
Choi YJ, Beakes G, Glockling S, Kruse J, Nam B, Nigrelli L, Ploch S, Shin HD, Shivas RG, Telle S. Towards a universal barcode of oomycetes—a comparison of the cox1 and cox2 loci. Mol Ecol Resour. 2015;15:1275–88.
Goss-Souza D, Mendes LW, Rodrigues JLM, Tsai SM. Ecological processes shaping bulk soil and rhizosphere microbiome assembly in a long-term amazon forest-to-agriculture conversion. Micro Ecol. 2020;79:110–22.
Hartmann M, Frey B, Mayer J, Mäder P, Widmer F. Distinct soil microbial diversity under long-term organic and conventional farming. ISME J. 2015;9:1177–94.
Nelkner J, Henke C, Lin TW, Pätzold W, Hassa J, Jaenicke S, Grosch R, Pühler A, Sczyrba A, Schlüter A. Effect of long-term farming practices on agricultural soil microbiome members represented by metagenomically assembled genomes (MAGs) and their predicted plant-beneficial genes. Genes. 2019;10:424.
Busby PE, Soman C, Wagner MR, Friesen ML, Kremer J, Bennett A, Morsy M, Eisen JA, Leach JE, Dangl JL. Research priorities for harnessing plant microbiomes in sustainable agriculture. PLoS Biol. 2017;15:e2001793.
Babin D, Deubel A, Jacquiod S, Sørensen SJ, Geistlinger J, Grosch R, Smalla K. Impact of long-term agricultural management practices on soil prokaryotic communities. Soil Biol Biochem. 2019;129:17–28.
Rachid CT, Balieiro FC, Fonseca ES, Peixoto RS, Chaer GM, Tiedje JM, Rosado AS. Intercropped silviculture systems, a key to achieving soil fungal community management in Eucalyptus plantations. PLoS One. 2015;10:e0118515.
•• Pereira APA, Zagatto MRG, Brandani CB, Mescolotti DDL, Cotta SR, Gonçalves JLM, Cardoso EJBN. Acacia changes microbial indicators and increases C and N in soil organic fractions in intercropped Eucalyptus plantations. Front Microbiol. 2018;9:655 This study found that intercropping one mycorrhizal and one rhizobial species had a beneficial effect on soil health. Acacia mangium improves the dynamics of soil microbial indicators, assisting in the accumulation of C and N in intercropped Eucalyptus grandis.
Pereira APA, Durrer A, Gumiere T, Gonçalves JLM, Robin A, Bouillet J-P, Wang J, Verma JP, Singh BK, Cardoso EJBN. Mixed Eucalyptus plantations induce changes in microbial communities and increase biological functions in the soil and litter layers. Forest Ecol Manag. 2019;433:332–42.
de Araujo Pereira AP, Santana MC, Verma JP. Mixed plantations of Eucalyptus and leguminous trees: Soil, microbiology, and ecosystem services. Anthro Sci. 2021;1:226–8.
Burgess AJ, Correa Cano ME, Parkes B. The deployment of intercropping and agroforestry as adaptation to climate change. Crop Environ. 2022;1:145–60.
Maharani D, Sudomo A, Swestiani D, Murniati SGE, Roshetko JM, Fambayun RA. Intercropping tuber crops with teak in Gunungkidul Regency, Yogyakarta, Indonesia. Agronomy. 2022;12:449.
Nadir SW, Ng'etich WK, Kebeney SJ. Performance of crops under Eucalyptus tree-crop mixtures and its potential for adoption in agroforestry systems. Aust J Crop Sci. 2018;12:1231–40.
Couto L, Gomes JM, Binkley D, Betters DR, Passos CAM. Intercropping eucalypts with beans in Minas Gerais Brazil. Int Tree Crops J. 1995;8:83–93.
Homulle Z, George TS, Karley AJ. Root traits with team benefits: understanding belowground interactions in intercropping systems. Plant Soil. 2022;471:1–26.
Yu R-P, Yang H, Xing Y, Zhang W-P, Lambers H, Li L. Belowground processes and sustainability in agroecosystems with intercropping. Plant oil. 2022;476:263–88.
Turner TR, James EK, Poole PS. The plant microbiome. Genome Biol. 2013;14:209.
Berg G, Grube M, Schloter M, Smalla K. Unraveling the plant microbiome: looking back and future perspectives. Front Microbiol. 2014;5:148.
Bulgarelli D, Schlaeppi K, Spaepen S, Van Themaat EVL, Schulze-Lefert P. Structure and functions of the bacterial microbiota of plants. Ann Rev Plant Biol. 2013;64:807–38.
Redford AJ, Bowers RM, Knight R, Linhart Y, Fierer N. The ecology of the phyllosphere: geographic and phylogenetic variability in the distribution of bacteria on tree leaves. Environ Microbiol. 2010;12:2885–93.
Kim M, Singh D, Lai-Hoe A, Go R, Rahim RA, Ainuddin A, Chun J, Adams JM. Distinctive phyllosphere bacterial communities in tropical trees. Micro Ecol. 2012;63:674–81.
Großkopf T, Soyer OS. Synthetic microbial communities. Curr Opin Microbiol. 2014;18:72–7.
Vorholt JA, Vogel C, Carlström CI, Müller DB. Establishing causality: opportunities of synthetic communities for plant microbiome research. Cell Host Microbe. 2017;22:142–55.
Bodenhausen N, Bortfeld-Miller M, Ackermann M, Vorholt JA. A synthetic community approach reveals plant genotypes affecting the phyllosphere microbiota. PLoS Gen. 2014;10:e1004283.
Kwak M-J, Kong HG, Choi K, Kwon S-K, Song JY, Lee J, Lee PA, Choi SY, Seo M, Lee HJ. Rhizosphere microbiome structure alters to enable wilt resistance in tomato. Nat Biotechnol. 2018;36:1100–9.
Niu B, Paulson JN, Zheng X, Kolter R. Simplified and representative bacterial community of maize roots. Proc Nat Acad Sci. 2017;114:E2450–E9.
Herrera Paredes S, Gao T, Law TF, Finkel OM, Mucyn T, Teixeira PJPL, Salas González I, Feltcher ME, Powers MJ, Shank EA, Jones CD, Jojic V, Dangl JL, Castrillo G. Design of synthetic bacterial communities for predictable plant phenotypes. PLoS Biol. 2018;16:e2003962.
Li S-p, Tan J, Yang X, Ma C, Jiang L. Niche and fitness differences determine invasion success and impact in laboratory bacterial communities. ISME J. 2019;13:402–12.
Mallon CA, Le Roux X, van Doorn GS, Dini-Andreote F, Poly F, Salles JF. The impact of failure: unsuccessful bacterial invasions steer the soil microbial community away from the invader’s niche. ISME J. 2018;12:728–41.
Kinnunen M, Dechesne A, Proctor C, Hammes F, Johnson D, Quintela-Baluja M, Graham D, Daffonchio D, Fodelianakis S, Hahn N, Boon N, Smets BF. A conceptual framework for invasion in microbial communities. ISME J. 2016;10:2773–9.
Mallon CA, Van Elsas JD, Salles JF. Microbial invasions: the process, patterns, and mechanisms. Trends Microbiol. 2015;23:719–29.
Wei Z, Yang T, Friman V-P, Xu Y, Shen Q, Jousset A. Trophic network architecture of root-associated bacterial communities determines pathogen invasion and plant health. Nat Commun. 2015;6:8413.
Litchman E. Invisible invaders: non-pathogenic invasive microbes in aquatic and terrestrial ecosystems. Ecol Lett. 2010;13:1560–72.
Thakur MP, van der Putten WH, Cobben MMP, van Kleunen M, Geisen S. Microbial invasions in terrestrial ecosystems. Nat Rev Microbiol. 2019;17:621–31.
Padmanaba M, Corlett RT. Minimizing risks of invasive alien plant species in tropical production forest management. Forests. 2014;5:1982–98.
Jin D, Huang Y, Zhou X-L, Chen B, Ma J, Yan Y-H. High risk of plant invasion in the understory of eucalypt plantations in South China. Sci Rep. 2015;5:18492.
Elsheikh EAE, El-Keblawy A, Mosa KA, Okoh AI, Saadoun I. Role of endophytes and rhizosphere microbes in promoting the invasion of exotic plants in arid and semi-arid areas: a review. Sustain. 2021;13:13081.
Aslani F, Juraimi AS, Ahmad-Hamdani MS, Alam MA, Hasan MM, Hashemi FSG, Bahram M. The role of arbuscular mycorrhizal fungi in plant invasion trajectory. Plant Soil. 2019;441:1–14.
Callaway RM, Thelen GC, Rodriguez A, Holben WE. Soil biota and exotic plant invasion. Nature. 2004;427:731–3.
Van der Putten WH. Impacts of soil microbial communities on exotic plant invasions. Trends Ecol Evol. 2010;25:512–9.
Gareca EE, Martinez YY, Bustamante RO, Aguirre LF, Siles MM. Regeneration patterns of Polylepis subtusalbida growing with the exotic trees Pinus radiata and Eucalyptus globulus at Parque Nacional Tunari Bolivia. Plant Ecol. 2007;193:253–63.
Xu Z, Zuo L, Zhang Y, Huang R, Li L. Is allelochemical synthesis in Casuarina equisetifolia plantation related to litter microorganisms? Front Plant Sci. 2022;13:1022984.
Wang Q, Wang S, Huang Y. Leaf litter decomposition in the pure and mixed plantations of Cunninghamia lanceolata and Michelia macclurei in subtropical China. Biol Fert Soils. 2009;45:371–7.
Kerry B. Fungal parasites of cyst nematodes. Agricult , Ecosyst Environ. 1988;24:293–305.
Gair R, Mathias P, Harvey P. Studies of cereal nematode populations and cereal yields under continuous or intensive culture. Ann Appl Biol. 1969;63:503–12.
Hayden HL, Savin KW, Wadeson J, Gupta VV, Mele PM. Comparative metatranscriptomics of wheat rhizosphere microbiomes in disease suppressive and non-suppressive soils for Rhizoctonia solani AG8. Front Microbiol. 2018;9:859.
Wiseman BM, Neate SM, Keller KO, Smith SE. Suppression of Rhizoctonia solani anastomosis group 8 in Australia and its biological nature. Soil Biol Biochem. 1996;28:727–32.
Kluepfel D, McInnis T, Zehr E. Involvement of root-colonizing bacteria in peach orchard soils suppressive of the nematode Criconemella xenoplax. Phytopathology. 1993;83:1240.
Hunjan MS, Sabhikhi HS. Designing a crop rotation strategy to manage Streptomyces scabies causing potato scab in north India. J Phytopathol. 2020;168:469–77.
Menzies JD. Occurrence and transfer of abiological factor in soil that suppresses potato scab. Phytopathology. 1959;49:648–52.
Runno-Paurson E, Lääniste P, Eremeev V, Tähtjärv T, Kaurilind E, Tosens T, Niinemets Ü, Williams IH. Does winter oilseed rape as a winter cover crop influence potato late blight development in an organic crop rotation? Biol Agricult Horticult. 2020;36:71–83.
Andrivon D. Dynamics of the survival and infectivity to potato tubers of sporangia of Phytophthora infestans in three different soils. Soil Biol Biochem. 1994;26:945–52.
Shiomi Y, Nishiyama M, Onizuka T, Marumoto T. Comparison of bacterial community structures in the rhizoplane of tomato plants grown in soils suppressive and conducive towards bacterial wilt. Appl Environ Microbiol. 1999;65:3996–4001.
Weibelzahl-Fulton E, Dickson D, Whitty E. Suppression of Meloidogyne incognita and M. javanica by Pasteuria penetrans in field soil. J Nematol. 1996;28:43.
Fortnum B, Lewis S, Johnson A. Crop rotation and nematicides for management of mixed populations of Meloidogyne spp. on tobacco. J Nematol. 2001;33:318–24.
Burke D, Kraft J. Responses of beans and peas to root pathogens accumulated during monoculture of each crop species. Phytopathology. 1974;64:546–9.
Alabouvette C, Lemanceau P, Steinberg C. Recent advances in the biological control of Fusarium wilts. Pest Sci. 1993;37:365–73.
Henis Y, Ghaffar A, Baker R. Factors affecting suppressiveness to Rhizoctonia solani in soil. Phytopathology. 1979;69:1164–9.
Murakami H, Tsushima S, Shishido Y. Soil suppressiveness to clubroot disease of Chinese cabbage caused by Plasmodiophora brassicae. Soil Biol Biochem. 2000;32:1637–42.
Alabouvette C. Fusarium-wilt suppressive soils from the Châteaurenard region: review of a 10-year study. Agronomie. 1986;6:273–84.
Persson L, Larsson-Wikström M, Gerhardson B. Assessment of soil suppressiveness to Aphanomyces root rot of pea. Plant Dis. 1999;83:1108–12.
Cha J-Y, Han S, Hong H-J, Cho H, Kim D, Kwon Y, Kwon S-K, Crüsemann M, Lee YB, Kim JF. Microbial and biochemical basis of a Fusarium wilt-suppressive soil. ISME J. 2016;10:119–29.
Crump DH, Kerry BR. Studies on the population dynamics and fungal parasitism of Heterodera schachtii in soil from a sugar-beet monoculture. Crop Protect. 1987;6:49–55.
Westphal A, Becker J. Biological suppression and natural population decline of Heterodera schachtii in a California field. Phytopathology. 1999;89:434–40.
Witt C, Cassman K, Olk D, Biker U, Liboon S, Samson M, Ottow J. Crop rotation and residue management effects on carbon sequestration, nitrogen cycling and productivity of irrigated rice systems. Plant Soil. 2000;225:263–78.
Acknowledgements
Dr Andi Wilson (FABI) provided substantial assistance in revising an early version of the manuscript and Prof Johanna Witzell, Department of Forestry and Wood Technology, Linnaeus University, added insightful suggestions during the review process. The authors thank the illustrators who generously made their images publicly available through Freepik (https://www.freepik.com/ ), allowing us to create the artwork for this article.
Funding
Open access funding provided by University of Pretoria. This work was financially supported by the University of Pretoria and the Tree Protection Cooperative Programme (TPCP), Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, South Africa.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bose, T., Hammerbacher, A., Slippers, B. et al. Continuous Replanting Could Degrade Soil Health in Short-Rotation Plantation Forestry. Curr. For. Rep. 9, 230–250 (2023). https://doi.org/10.1007/s40725-023-00188-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40725-023-00188-z