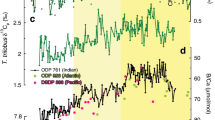
Abstract
Phosphorus is a limiting nutrient that is thought to control oceanic oxygen levels to a large extent1,2,3. A possible increase in marine phosphorus concentrations during the Ediacaran Period (about 635–539 million years ago) has been proposed as a driver for increasing oxygen levels4,5,6. However, little is known about the nature and evolution of phosphorus cycling during this time4. Here we use carbonate-associated phosphate (CAP) from six globally distributed sections to reconstruct oceanic phosphorus concentrations during a large negative carbon-isotope excursion—the Shuram excursion (SE)—which co-occurred with global oceanic oxygenation7,8,9. Our data suggest pulsed increases in oceanic phosphorus concentrations during the falling and rising limbs of the SE. Using a quantitative biogeochemical model, we propose that this observation could be explained by carbon dioxide and phosphorus release from marine organic-matter oxidation primarily by sulfate, with further phosphorus release from carbon-dioxide-driven weathering on land. Collectively, this may have resulted in elevated organic-pyrite burial and ocean oxygenation. Our CAP data also seem to suggest equivalent oceanic phosphorus concentrations under maximum and minimum extents of ocean anoxia across the SE. This observation may reflect decoupled phosphorus and ocean anoxia cycles, as opposed to their coupled nature in the modern ocean. Our findings point to external stimuli such as sulfate weathering rather than internal oceanic phosphorus–oxygen cycling alone as a possible control on oceanic oxygenation in the Ediacaran. In turn, this may help explain the prolonged rise of atmospheric oxygen levels.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All data generated or analysed during this study are available at https://figshare.com/articles/dataset/Dodd_et_al_2023_xlsx/22274293 and included with the published article (and its Supplementary Information files). Source data are provided with this paper.
Code availability
MATLAB code for COPSE is freely available at https://github.com/bjwmills/COPSE.
References
Van Cappellen, P. & Ingall, E. D. Redox stabilization of the atmosphere and oceans by phosphorus-limited marine productivity. Science 271, 493–496 (1996).
Algeo, T. J. & Ingall, E. Sedimentary Corg:P ratios, paleocean ventilation, and Phanerozoic atmospheric pO2. Palaeogeogr. Palaeoclimatol. Palaeoecol. 256, 130–155 (2007).
Slomp, C. P. & Van Cappellen, P. The global marine phosphorus cycle: sensitivity to oceanic circulation. Biogeosciences 4, 155–171 (2007).
Laakso, T. A., Sperling, E. A., Johnston, D. T. & Knoll, A. H. Ediacaran reorganization of the marine phosphorus cycle. Proc. Natl Acad. Sci. 117, 11961 (2020).
Kipp, M. A. & Stüeken, E. E. Biomass recycling and Earth’s early phosphorus cycle. Sci. Adv. 3, eaao4795 (2017).
Reinhard, C. T. et al. Evolution of the global phosphorus cycle. Nature 541, 386–389 (2017).
Zhang, F. et al. Extensive marine anoxia during the terminal Ediacaran Period. Sci. Adv. 4, eaan8983 (2018).
Zhang, F. et al. Global marine redox changes drove the rise and fall of the Ediacara biota. Geobiology 17, 594–610 (2019).
Tostevin, R. et al. Uranium isotope evidence for an expansion of anoxia in terminal Ediacaran oceans. Earth Planet. Sci. Lett. 506, 104–112 (2019).
Ruttenberg, K. C. in Treatise on Geochemistry Vol. 8 (eds Holland, H. D. & Turekian, K. K.) 585–643 (Pergamon, 2003).
Ingall, E. D., Bustin, R. M. & Van Cappellen, P. Influence of water column anoxia on the burial and preservation of carbon and phosphorus in marine shales. Geochim. Cosmochim. Acta 57, 303–316 (1993).
Xiong, Y. et al. Phosphorus cycling in Lake Cadagno, Switzerland: a low sulfate euxinic ocean analogue. Geochim. Cosmochim. Acta 251, 116–135 (2019).
Alcott, L. J., Mills, B. J. W. & Poulton, S. W. Stepwise Earth oxygenation is an inherent property of global biogeochemical cycling. Science 366, 1333–1337 (2019).
Planavsky, N. J. et al. The evolution of the marine phosphate reservoir. Nature 467, 1088–1090 (2010).
Bjerrum, C. J. & Canfield, D. E. Ocean productivity before about 1.9 Gyr ago limited by phosphorus adsorption onto iron oxides. Nature 417, 159–162 (2002).
Jones, C., Nomosatryo, S., Crowe, S. A., Bjerrum, C. J. & Canefield, D. E. Iron oxides, divalent cations, silica, and the early earth phosphorus crisis. Geology 43, 135–138 (2015).
Derry, L. A. Causes and consequences of mid-Proterozoic anoxia. Geophys. Res. Lett. 42, 8538–8546 (2015).
Evans, S. D., Diamond, C. W., Droser, M. L. & Lyons, T. W. Dynamic oxygen and coupled biological and ecological innovation during the second wave of the Ediacara Biota. Emerg. Top. Life Sci. 2, 223–233 (2018).
Darroch, S. A. F., Smith, E. F., Laflamme, M. & Erwin, D. H. Ediacaran extinction and Cambrian explosion. Trends Ecol. Evol. 33, 653–663 (2018).
Fike, D. A., Grotzinger, J. P., Pratt, L. M. & Summons, R. E. Oxidation of the Ediacaran ocean. Nature 444, 744–747 (2006).
Shi, W. et al. Sulfur isotope evidence for transient marine-shelf oxidation during the Ediacaran Shuram Excursion. Geology 46, 267–270 (2018).
Li, C. et al. Uncovering the spatial heterogeneity of Ediacaran carbon cycling. Geobiology 15, 211–224 (2017).
Kaufman, A. J., Corsetti, F. A. & Varni, M. A. The effect of rising atmospheric oxygen on carbon and sulfur isotope anomalies in the Neoproterozoic Johnnie Formation, Death Valley, USA. Chem. Geol. 237, 47–63 (2007).
Loyd, S. J. et al. Sustained low marine sulfate concentrations from the Neoproterozoic to the Cambrian: insights from carbonates of northwestern Mexico and eastern California. Earth Planet. Sci. Lett. 339–340, 79–94 (2012).
Merdith, A. S. et al. A full-plate global reconstruction of the Neoproterozoic. Gondwana Res. 50, 84–134 (2017).
Huang, K. et al. Interaction of Shibantan Biota and environment in the terminal Ediacaran ocean: evidence from I/(Ca+Mg) and sulfur isotopes. Precambrian Res. 379, 106814 (2022).
Wang, R. et al. A great late Ediacaran ice age. Natl Sci. Rev. https://doi.org/10.1093/nsr/nwad117 (2023).
Rooney, A. D. et al. Calibrating the coevolution of Ediacaran life and environment. Proc. Natl Acad. Sci. 117, 16824–16830 (2020).
Dodd, M. S. et al. Development of carbonate-associated phosphate (CAP) as a proxy for reconstructing ancient ocean phosphate levels. Geochim. Cosmochim. Acta 301, 48–69 (2021).
Shimura, T. et al. In-situ analyses of phosphorus contents of carbonate minerals: reconstruction of phosphorus contents of seawater from the Ediacaran to early Cambrian. Gondwana Res. 25, 1090–1107 (2014).
Swart, P. K. & Kennedy, M. J. Does the global stratigraphic reproducibility of δ13C in Neoproterozoic carbonates require a marine origin? A Pliocene–Pleistocene comparison. Geology 40, 87–90 (2012).
Busch, J. F. et al. Global and local drivers of the Ediacaran Shuram carbon isotope excursion. Earth Planet. Sci. Lett. 579, 117368 (2022).
Loyd, S. J., Berelson, W. M., Lyons, T. W., Hammond, D. E. & Corsetti, F. A. Constraining pathways of microbial mediation for carbonate concretions of the Miocene Monterey Formation using carbonate-associated sulfate. Geochim. Cosmochim. Acta 78, 77–98 (2012).
Cheng, M. et al. Barite in the Ediacaran Doushantuo Formation and its implications for marine carbon cycling during the largest negative carbon isotope excursion in Earth’s history. Precambrian Res. 368, 106485 (2022).
Hardisty, D. S. et al. Perspectives on Proterozoic surface ocean redox from iodine contents in ancient and recent carbonate. Earth Planet. Sci. Lett. 463, 159–170 (2017).
Wood, R., Bowyer, F., Penny, A. & Poulton, S. W. Did anoxia terminate Ediacaran benthic communities? Evidence from early diagenesis. Precambrian Res. 313, 134–147 (2018).
Shields, G. A. et al. Unique Neoproterozoic carbon isotope excursions sustained by coupled evaporite dissolution and pyrite burial. Nat. Geosci. 12, 823–827 (2019).
Rothman, D. H., Hayes, J. M. & Summons, R. E. Dynamics of the Neoproterozoic carbon cycle. Proc. Natl Acad. Sci. 100, 8124 (2003).
Bjerrum, C. J. & Canfield, D. E. Towards a quantitative understanding of the late Neoproterozoic carbon cycle. Proc. Natl Acad. Sci. 108, 5542 (2011).
Derry, L. A. A burial diagenesis origin for the Ediacaran Shuram–Wonoka carbon isotope anomaly. Earth Planet. Sci. Lett. 294, 152–162 (2010).
Sawaki, Y. et al. The Ediacaran radiogenic Sr isotope excursion in the Doushantuo Formation in the Three Gorges area, South China. Precambrian Res. 176, 46–64 (2010).
Wei, G.-Y. et al. Long-term evolution of terrestrial inputs from the Ediacaran to early Cambrian: clues from Nd isotopes in shallow-marine carbonates, South China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 535, 109367 (2019).
Li, C. et al. A stratified redox model for the Ediacaran ocean. Science 328, 80 (2010).
Canfield, D. E. et al. Ferruginous conditions dominated later Neoproterozoic deep-water chemistry. Science 321, 949 (2008).
Li, Z. et al. Transient and stepwise ocean oxygenation during the late Ediacaran Shuram Excursion: insights from carbonate δ238U of northwestern Mexico. Precambrian Res. 344, 105741 (2020).
Fan, H. et al. Constraining oceanic oxygenation during the Shuram excursion in South China using thallium isotopes. Geobiology 18, 348–365 (2020).
Kendall, B. et al. Uranium and molybdenum isotope evidence for an episode of widespread ocean oxygenation during the late Ediacaran Period. Geochim. Cosmochim. Acta 156, 173–193 (2015).
Jin, C. et al. Highly heterogeneous “poikiloredox” conditions in the early Ediacaran Yangtze Sea. Precambrian Res. 311, 157–166 (2018).
Lyons, T. W., Reinhard, C. T. & Planavsky, N. J. The rise of oxygen in Earth’s early ocean and atmosphere. Nature 506, 307–315 (2014).
Alcott, L. J., Mills, B. J. W., Bekker, A. & Poulton, S. W. Earth’s Great Oxidation Event facilitated by the rise of sedimentary phosphorus recycling. Nat. Geosci. 15, 210–215 (2022).
Cui, H. et al. Redox architecture of an Ediacaran ocean margin: integrated chemostratigraphic (δ13C–δ34S–87Sr/86Sr–Ce/Ce*) correlation of the Doushantuo Formation, South China. Chem. Geol. 405, 48–62 (2015).
Bellefroid, E. J., Planavsky, N. J., Miller, N. R., Brand, U. & Wang, C. Case studies on the utility of sequential carbonate leaching for radiogenic strontium isotope analysis. Chem. Geol. 497, 88–99 (2018).
Zhou, F.-Y. et al. Development of an automatic column chromatography separation device for metal isotope analysis based on droplet counting. Anal. Chem. 93, 7196–7203 (2021).
Barkan, Y., Paris, G., Webb, S. M., Adkins, J. F. & Halevy, I. Sulfur isotope fractionation between aqueous and carbonate-associated sulfate in abiotic calcite and aragonite. Geochim. Cosmochim. Acta 280, 317–339 (2020).
Lenton, T. M., Daines, S. J. & Mills, B. J. W. COPSE reloaded: an improved model of biogeochemical cycling over Phanerozoic time. Earth Sci. Rev. 178, 1–28 (2018).
Brüchert, V. et al. Regulation of bacterial sulfate reduction and hydrogen sulfide fluxes in the central Namibian coastal upwelling zone. Geochim. Cosmochim. Acta 67, 4505–4518 (2003).
Acknowledgements
We thank B. Shen and R. Wang for generously providing samples from the Mochia-Khutuk section and L. Zheng for assistance in obtaining the Sishang section samples. This study was supported by the NSFC (grant nos. 41825019, 42130208 and 41821001) and the National Key Research and Development Program of China (2022YFF0800100) for funding. M.S.D. acknowledges support from the International Exchange Program for Postdoctors of China and funding from the China Postdoctoral Science Foundation, the Forrest Research Foundation and the University of Western Australia School of Earth Sciences. A.vS.H. and M.W. acknowledge support from the ARC (DE190100988 and DP210103715). Further funding through the NASA Astrobiology Institute under Cooperative Agreement No. NNA15BB03A issued through the Science Mission Directorate and the Interdisciplinary Consortia for Astrobiology Research (T.W.L.).
Author information
Authors and Affiliations
Contributions
C.L. led the research. C.L. and M.S.D. designed the research. M.S.D., Z.Z., M.C. and H.G. performed analyses. W.S. and B.J.W.M. conducted modelling work. C.L., T.W.L., D.S.H., S.J.L., M.W.W., A.vS.H., K.L., M.C. and H.G. provided samples and assistance in the field. S.W.P. provided analytical assistance. M.S.D., C.L. and W.S. wrote the manuscript, with important discussion and contributions from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Open-system diagenetic evolution fluid–rock interaction model.
a–c, Fluid–rock alteration models showing the relative order of alteration for CAP, CAS, Fe, Mn, IO3, δ13C, δ34SCAS, δ238U, δ44/40Ca and 87Sr/86Sr. Several curves are presented for δ13C and δ34SCAS under varying DIC and sulfate concentrations in the diagenetic fluid. d, Fluid–rock alteration model showing the predicted trends between CAP and δ13C. Grey points are CAP and δ13C data from all study sections. Solid and dashed lines represent different pore-water DIC concentration and δ13C compositions. Dotted line is trendline through data points with R value. See Supplementary Information for model description. Yellow stars mark the point at which 50% of the CAP value has been altered. Red stars mark the point at which 50% of the element of interest has been altered.
Extended Data Fig. 2 COPSE model results comparing different hypotheses (DOM oxidation by sulfate only, elevated organic-matter recycling, elevated weathering by uplift, elevated weathering by volcanism) for the observed changes in ocean P during the SE.
a,A,I,i, Relative increase in sulfate addition versus background flux. b,B,II,ii, Phosphorus concentration in seawater ([P]sw). c,C,III,iii, Relative atmospheric oxygen concentration (pO2). d,D,IV,iv, Degree of ocean anoxia (Anoxia). e,E,V,v, Modelled marine carbonate carbon-isotope composition (δ13Ccarb). Note that in panel E, the δ13Ccarb reflects the δ13C of pore water DIC, not oceanic δ13C. f,F,VI,vi, Modelled marine sulfate sulfur-isotope composition (δ34Ssulfate). g,G,VII,vii, Modelled marine carbonate uranium-isotope composition (δ238Ucarb). For the DOM oxidation hypothesis, we run the COPSE model with a DOM reservoir 30 times the size of the modern marine DIC reservoir and the C:P of the DOM reservoir is 1,000, whereas the C:P of organic matter in the organic-matter recycle model is 250 (see Supplementary Information 6 for more details). PAL, present atmospheric level.
Extended Data Fig. 3 COPSE model results comparing the oxidation of a DOM reservoir using sulfate, free oxygen (Shields et al.37) and sulfate + free oxygen, respectively.
a,A,I, Relative increase in sulfate addition versus background flux (the varying colour shades of the model lines reflect the varying magnitudes of the sulfate pulse for different model runs). b,B,II, Phosphorus concentration in seawater ([P]sw). c,C,III, Relative atmospheric oxygen concentration (pO2). d,D,IV, Degree of ocean anoxia (Anoxia). e,E,V, Modelled marine carbonate carbon-isotope composition (δ13Ccarb). f,F,VI, Modelled marine sulfate sulfur-isotope composition (δ34Ssulfate). The C:P of the DOM reservoir is set to 1,000 in all model runs. The magnitude of the sulfate pulses for each model is variable because higher additional sulfate fluxes are required for models in which DOM is oxidized by O2 resulting from pyrite burial in comparison with models in which DOM is oxidized only by sulfate (see Supplementary Information 7 for details).
Extended Data Fig. 4 COPSE model results with varying sizes (A–F) and variable P content (a–d) of an initial DOM reservoir and higher initial steady-state pO2 of 20% present atmospheric level (PAL), with C:P of DOM = 250 (a–d) (no Fe2+-P burial) (I–IV).
A, Size of DOM reservoir in moles of carbon. B, Phosphorus concentration in seawater ([P]sw). C, Relative atmospheric oxygen concentration (pO2). D, Degree of ocean anoxia (Anoxia). E, Modelled marine carbonate carbon-isotope composition (δ13Ccarb). F, Modelled marine carbonate uranium-isotope composition (δ238Ucarb). In panels A–F, we choose a sulfate pulse of four times that of the background flux and the C:P of the DOM reservoir is 1,000. a,I, Size of DOM reservoir in moles of carbon. b,II, Phosphorus concentration in seawater ([P]sw). c,III, Relative atmospheric oxygen concentration (pO2). d,IV, Degree of ocean anoxia. In panels a–d, we choose a sulfate input of four times the background flux and the size of the DOM reservoir is 30 times that of the size of the modern marine DIC reservoir. For panels I–IV, the DOM reservoir is 30 times that of the size of the modern marine DIC. Higher steady-state pO2 was achieved by adjusting the model terrestrial P-input flux and gypsum burial.
Extended Data Fig. 5 COPSE model results varying the magnitude of a further sulfate pulse for DOM oxidation by sulfate only (no Fe2+-P burial) (a–h), burying all the extra sulfate pulse as pyrite (I–VIII) and setting gypsum burial to a constant rate (i–viii).
a, Size of DOM reservoir in moles of carbon. b, Modelled marine carbonate carbon-isotope composition (δ13Ccarb). c, Phosphorus concentration in seawater ([P]sw). d, Modelled marine carbonate uranium-isotope composition (δ238Ucarb). e, Relative atmospheric oxygen concentration (pO2). f, Degree of ocean anoxia. g, Modelled marine sulfate sulfur-isotope composition (δ34Ssulfate). h, Modelled marine carbonate strontium-isotope composition (87Sr/86Sr). The blue line, grey line and dashed grey line are sulfate pulses of three, four and five times the background flux, respectively. I,i, Relative atmospheric oxygen concentration (pO2). II,ii, Phosphorus concentration in seawater ([P]sw). III,iii, Modelled marine carbonate carbon-isotope composition (δ13Ccarb). VI,vi, Evolution of ocean anoxia. V,v, Modelled marine carbonate uranium-isotope composition (δ238Ucarb). VI,vi, Modelled marine sulfate sulfur-isotope composition (δ34Ssulfate). VII,vii, Sulfate concentration in seawater ([SO4]sw). VIII,viii, Oceanic gypsum burial rate (mgsb). Here we used a DOM reservoir size that is 30 times that of the size of the modern marine DIC reservoir. PAL, present atmospheric level; POL, present oceanic level.
Extended Data Fig. 6 Full COPSE model outputs for DOM oxidation by sulfate with constant Fe2+-bound phosphorus burial, using a sulfate pulse of four times the background flux, DOM C:P of 1,000 and the size of the DOM reservoir is 30 times the size of the modern marine DIC reservoir.
a, Weathering sulfate pulse versus background flux. b, DOM oxidation flux (DOMox) in moles of carbon per year. c, DOM reservoir (DOMpool) in moles of carbon. d, P concentration in seawater ([P]sw). e, Relative atmospheric oxygen concentration (pO2) to present atmospheric level (PAL). f, Degree of marine anoxia (Anoxia). g, Modelled marine carbonate carbon-isotope composition (δ13Ccarb). h, Modelled marine sulfate sulfur-isotope composition (δ34Ssulfate). i, Silicate weathering flux (silw) in moles of carbon per year. j, Modelled marine carbonate strontium-isotope composition (87Sr/86Sr). k, Relative marine sulfate concentration ([SO42−]sw) to present oceanic level (POL). l, Relative marine new primary productivity (newp) to POL. m, Relative atmospheric carbon dioxide concentration (pCO2) to PAL. n, Average global temperature (Temp) in °C. o, Organic carbon weathering flux (oxidw) in moles of carbon per year. p, Marine organic carbon burial flux (mocb) in moles of carbon. q, Gypsum sulfur weathering flux (gypw) in moles of sulfur. r, Pyrite sulfur weathering flux (pyrw) in moles of sulfur. s, Marine pyrite sulfur burial flux (mpsb) in moles of sulfur. t, Marine gypsum sulfur burial flux (mgsb) in moles of sulfur. u, Phosphorus releasing flux from DOM oxidation (DOMOX_P) in moles of phosphorus. v, Flux of weathered phosphorus reaching the sea (psea) in moles of phosphorus. w, Total iron-bound phosphorus burial flux (fepb) in moles of phosphorus. x, Carbonate-bound phosphorus burial flux (capb) in moles of phosphorus. y, Marine organic phosphorus burial flux (mopb) in moles of phosphorus. z, Ferric iron Fe3+-bound phosphorus burial [fepb(Fe3+)] in moles of phosphorus. aa, Ferrous iron Fe2+-bound phosphorus burial [fepb(Fe2+)] in moles of phosphorus.
Extended Data Fig. 7 Comparison of COPSE model results for ocean P cycling with and without P burial by Fe2+ scavenging.
a, Ocean inorganic carbon isotopic composition (δ13Ccarb). b, Ocean P concentration ([P]sw). c, Ocean uranium isotopic composition recorded in carbonates (δ238Ucarb). d, Ocean sulfur isotopic composition recorded in carbonate-associated sulfate (δ34SCAS). e, Ocean strontium isotopic composition (87Sr/86Sr). f, Ediacaran fossil record adapted after Darroch et al.19. Stages I–IV are defined as the SE intervals of falling limb, plateau, rising limb and post-SE, respectively, as in Fig. 1, which are matched with modelled ocean P reservoir shifts. Model parameters for outputs are the same as detailed in Fig. 3 and Extended Data Fig. 6, except for the red line, which excludes P burial by Fe2+ scavenging (that is, a modern-style P and O2 cycle). P-1st and P-2nd refer to CAP peaks in Fig. 1.
Extended Data Fig. 8 Model output of a quantitative four-box ocean P cycle model.
Output from Figs. 4a and 5a in Alcott et al.13 with the P concentrations in the respective boxes (proximal shelf, distal shelf, deep ocean) plotted. This shows the relative concentration of soluble reactive phosphorus in each ocean box during a model solution in which P levels are oscillating on a large scale. These results show that, even under substantial changes in P concentration, the distal shelf (that is, the area of the shelf that is not dominated by riverine input) is expected to be strongly linked to the deep-ocean P concentration. See Alcott et al.13 for full model details.
Extended Data Fig. 9 Experimental constraints on the effects of alkalinity (a) and carbonate precipitation rate (b) on CAP values in carbonate.
a, CAP uptake increases with progressively lower [CO32−] and alkalinity concentrations. b, CAP uptake decreases with increasing precipitation rate. The changes in CAP over the observed ranges in alkalinity and precipitation rate are small compared with the effects of phosphate concentration and solution pH (Dodd et al.29). All trendlines are linear fits. Error bars are ±5% for CAP and ±0.1 for Ca/ALK.
Supplementary information
Supplementary Information
This file contains Supplementary Information 1–9, Supplementary Tables for COPSE and diagenetic models S1–S6, and Supplementary References.
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dodd, M.S., Shi, W., Li, C. et al. Uncovering the Ediacaran phosphorus cycle. Nature 618, 974–980 (2023). https://doi.org/10.1038/s41586-023-06077-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06077-6
This article is cited by
-
Uncovering the largest negative carbon isotope excursion in Earth history
Science China Earth Sciences (2024)
-
Sulfate triple-oxygen-isotope evidence confirming oceanic oxygenation 570 million years ago
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.