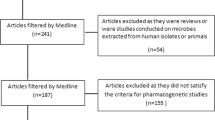
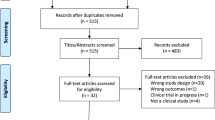
Abstract
The major challenges that delay the implementation of pharmacogenomics based clinical practice in the developing countries, primarily the low- and middle-income countries need to be recognized. This review was conducted to systematically review evidence of the cost-effectiveness for the conduct of pharmacogenomics testing in the developing countries. Studies that evaluated the cost-effectiveness of pharmacogenomics testing in the developing countries as defined by the United Nations were included in this study. Twenty-seven articles met the criteria. Pharmacogenomics effectiveness were evaluated for drugs used in the treatment of cancers, cardiovascular diseases and severe cutaneous adverse reactions in gout and epilepsy. Most studies had reported pharmacogenomics testing to be cost-effective (cancers, cardiovascular diseases, and tuberculosis) and economic models were evaluated from multiple perspectives, different cost categories and time horizons. Additionally, most studies used a single gene, rather than a gene panel for the pharmacogenomics testing. Genotyping cost and frequency of risk alleles in the populations influence the cost-effectiveness outcome. Further studies are warranted to examine the clinical and economic validity of pharmacogenomics testing in the developing countries.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Roden DM, McLeod HL, Relling MV, Williams MS, Mensah GA, Peterson JF, et al. Pharmacogenomics. Lancet. 2019;394:521–32.
Charlab R, Zhang L. Pharmacogenomics: historical perspective and current status. Methods Mol Biol. 2013;1015:3–22.
Juran BD, Egan LJ, Lazaridis KN. The AmpliChip CYP450 test: principles, challenges, and future clinical utility in digestive disease. Clin Gastroenterol Hepatol. 2006;4:822–30.
Pirmohamed M, Burnside G, Eriksson N, Jorgensen AL, Toh CH, Nicholson T, et al. A randomized trial of genotype-guided dosing of warfarin. N Engl J Med. 2013;369:2294–303.
Kleinjan JP, Brinkman I, Bakema R, van Zanden JJ, van Rooijen JM. Tolerance-based capecitabine dose escalation after DPYD genotype-guided dosing in heterozygote DPYD variant carriers: a single-center observational study. Anticancer Drugs. 2019;30:410–5.
Jung JW, Kim DK, Park HW, Oh KH, Joo KW, Kim YS, et al. An effective strategy to prevent allopurinol-induced hypersensitivity by HLA typing. Genet Med. 2015;17:807–14.
Askanase AD, Wallace DJ, Weisman MH, Tseng CE, Bernstein L, Belmont HM, et al. Use of pharmacogenetics, enzymatic phenotyping, and metabolite monitoring to guide treatment with azathioprine in patients with systemic lupus erythematosus. J Rheumatol. 2009;36:89–95.
Huerta-García G, Vazquez-Rosales JG, Mata-Marín JA, Peregrino-Bejarano L, Flores-Ruiz E, Solórzano-Santos F. Genotype-guided antiretroviral regimens in children with multidrug-resistant HIV-1 infection. Pediatr Res. 2016;80:54–9.
Nauman J, Soteriades ES, Hashim MJ, Govender R, Al Darmaki RS, Al, et al. Global incidence and mortality trends due to adverse effects of medical treatment, 1990-2017: a systematic analysis from the global burden of diseases, injuries and risk factors study. Cureus. 2020;12:e7265.
Patel TK, Patel PB. Mortality among patients due to adverse drug reactions that lead to hospitalization: a meta-analysis. Eur J Clin Pharm. 2018;74:819–32.
Sunshine JE, Meo N, Kassebaum NJ, Collison ML, Mokdad AH, Naghavi M. Association of adverse effects of medical treatment with mortality in the United States: a secondary analysis of the global burden of diseases, injuries, and risk factors study. JAMA Netw Open. 2019;2:e187041.
Zhang H, Du W, Gnjidic D, Chong S, Glasgow N. Trends in adverse drug reaction-related hospitalisations over 13 years in New South Wales, Australia. Intern Med J. 2019;49:84–93.
Angamo MT, Chalmers L, Curtain CM, Bereznicki LR. Adverse-drug-reaction-related hospitalisations in developed and developing countries: a review of prevalence and contributing factors. Drug Saf. 2016;39:847–57.
Ampadu HH, Hoekman J, de Bruin ML, Pal SN, Olsson S, Sartori D, et al. Adverse drug reaction reporting in Africa and a comparison of individual case safety report characteristics between Africa and the rest of the world: analyses of spontaneous reports in VigiBase®. Drug Saf. 2016;39:335–45.
Alshabeeb MA, Deneer VHM, Khan A, Asselbergs FW. Use of pharmacogenetic drugs by the Dutch population. Front Genet. 2019;10:567.
Giannopoulou E, Katsila T, Mitropoulou C, Tsermpini EE, Patrinos GP. Integrating next-generation sequencing in the clinical pharmacogenomics workflow. Front Pharm. 2019;10:384.
Schneider JA, Cohen PR. Stevens-Johnson syndrome and toxic epidermal necrolysis: a concise review with a comprehensive summary of therapeutic interventions emphasizing supportive measures. Adv Ther. 2017;34:1235–44.
Thornley T, Esquivel B, Wright DJ, Dop HVD, Kirkdale CL, Youssef E. Implementation of a pharmacogenomic testing service through community pharmacy in the Netherlands: results from an early service evaluation. Pharm (Basel). 2021;9:38.
B Tata E, A Ambele M, S Pepper M. Barriers to implementing clinical pharmacogenetics testing in sub-Saharan Africa. A critical review. Pharmaceutics/. 2020;12:809.
Deverka PA, Vernon J, McLeod HL. Economic opportunities and challenges for pharmacogenomics. Annu Rev Pharm Toxicol. 2010;50:423–37.
Nimdet K, Chaiyakunapruk N, Vichansavakul K, Ngorsuraches S. A systematic review of studies eliciting willingness-to-pay per quality-adjusted life year: does it justify CE threshold? PLoS ONE. 2015;10:e0122760.
Turner HC, Archer RA, Downey LE, Isaranuwatchai W, Chalkidou K, Jit M, et al. An introduction to the main types of economic evaluations used for informing priority setting and resource allocation in healthcare: key features, uses, and limitations. Front Public Health. 2021;9:722927.
Kim DD, Silver MC, Kunst N, Cohen JT, Ollendorf DA, Neumann PJ. Perspective and costing in cost-effectiveness analysis, 1974-2018. Pharmacoeconomics. 2020;38:1135–45.
Zhu Y, Swanson KM, Rojas RL, Wang Z, St Sauver JL, Visscher SL, et al. Systematic review of the evidence on the cost-effectiveness of pharmacogenomics-guided treatment for cardiovascular diseases. Genet Med. 2020;22:475–86.
United Nations. World Economic Situation and Prospects 2021. Available from: https://www.un.org/development/desa/dpad/wp-content/uploads/sites/45/WESP2021_ANNEX.pdf.
Wijnen B, Van Mastrigt G, Redekop WK, Majoie H, De Kinderen R, Evers S. How to prepare a systematic review of economic evaluations for informing evidence-based healthcare decisions: data extraction, risk of bias, and transferability (part 3/3). Expert Rev Pharmacoecon Outcomes Res. 2016;16:723–32.
Ofman JJ, Sullivan SD, Neumann PJ, Chiou CF, Henning JM, Wade SW, et al. Examining the value and quality of health economic analyses: implications of utilizing the QHES. J Manag Care Pharm. 2003;9:53–61.
Jiang M, You JH. Cost-effectiveness analysis of personalized antiplatelet therapy in patients with acute coronary syndrome. Pharmacogenomics. 2016;17:701–13.
Fu Y, Zhang XY, Qin SB, Nie XY, Shi LW, Shao H, et al. Cost-effectiveness of CYP2C19 LOF-guided antiplatelet therapy in Chinese patients with acute coronary syndrome. Pharmacogenomics. 2020;21:33–42.
Teng GG, Tan-Koi WC, Dong D, Sung C. Is HLA-B*58:01 genotyping cost effective in guiding allopurinol use in gout patients with chronic kidney disease? Pharmacogenomics. 2020;21:279–91.
Chong HY, Saokaew S, Dumrongprat K, Permsuwan U, Wu DB, Sritara P, et al. Cost-effectiveness analysis of pharmacogenetic-guided warfarin dosing in Thailand. Thromb Res. 2014;134:1278–84.
Wei X, Cai J, Sun H, Li N, Xu C, Zhang G, et al. Cost-effectiveness analysis of UGT1A1*6/*28 genotyping for preventing FOLFIRI-induced severe neutropenia in Chinese colorectal cancer patients. Pharmacogenomics. 2019;20:241–9.
Chen Z, Liew D, Kwan P. Real-world cost-effectiveness of pharmacogenetic screening for epilepsy treatment. Neurology. 2016;86:1086–94.
Wei X, Cai J, Zhuang J, Zheng B, Sui Y, Zhang G, et al. CYP2D6*10 pharmacogenetic-guided SERM could be a cost-effective strategy in Chinese patients with hormone receptor-positive breast cancer. Pharmacogenomics. 2020;21:43–53.
Wang Y, Yan BP, Liew D, Lee VWY. Cost-effectiveness of cytochrome P450 2C19 *2 genotype-guided selection of clopidogrel or ticagrelor in Chinese patients with acute coronary syndrome. Pharmacogenomics J. 2018;18:113–20.
Ke CH, Chung WH, Wen YH, Huang YB, Chuang HY, Tain YL, et al. Cost-effectiveness analysis for genotyping before allopurinol treatment to prevent severe cutaneous adverse drug reactions. J Rheumatol. 2017;44:835–43.
Kim DJ, Kim HS, Oh M, Kim EY, Shin JG. Cost Effectiveness of genotype-guided warfarin dosing in patients with mechanical heart valve replacement under the fee-for-service system. Appl Health Econ Health Policy. 2017;15:657–67.
Dong D, Tan-Koi WC, Teng GG, Finkelstein E, Sung C. Cost-effectiveness analysis of genotyping for HLA-B*5801 and an enhanced safety program in gout patients starting allopurinol in Singapore. Pharmacogenomics. 2015;16:1781–93.
Kim JH, Tan DS, Chan MYY. Cost-effectiveness of CYP2C19-guided antiplatelet therapy for acute coronary syndromes in Singapore. Pharmacogenomics J. 2021;21:243–50.
Lu S, Zhang J, Ye M, Wang B, Wu B. Economic analysis of ALK testing and crizotinib therapy for advanced non-small-cell lung cancer. Pharmacogenomics. 2016;17:985–94.
You JH, Chan FW, Wong RS, Cheng G. The potential clinical and economic outcomes of pharmacogenetics-oriented management of warfarin therapy—a decision analysis. Thromb Haemost. 2004;92:590–7.
de Lima Lopes G Jr, Segel JE, Tan DS, Do YK, Mok T, Finkelstein EA. Cost-effectiveness of epidermal growth factor receptor mutation testing and first-line treatment with gefitinib for patients with advanced adenocarcinoma of the lung. Cancer. 2012;118:1032–9.
Wei X, Sun H, Zhuang J, Weng X, Zheng B, Lin Q, et al. Cost-effectiveness Analysis of CYP2D6*10 pharmacogenetic testing to guide the adjuvant endocrine therapy for postmenopausal women with estrogen receptor positive early breast cancer in China. Clin Drug Investig. 2020;40:25–32.
Lu S, Yu Y, Fu S, Ren H. Cost-effectiveness of ALK testing and first-line crizotinib therapy for non-small-cell lung cancer in China. PLoS ONE. 2018;13:e0205827.
Kapoor R, Martinez-Vega R, Dong D, Tan SY, Leo YS, Lee CC, et al. Reducing hypersensitivity reactions with HLA-B*5701 genotyping before abacavir prescription: clinically useful but is it cost-effective in Singapore? Pharmacogenet Genomics. 2015;25:60–72.
Saokaew S, Tassaneeyakul W, Maenthaisong R, Chaiyakunapruk N. Cost-effectiveness analysis of HLA-B*5801 testing in preventing allopurinol-induced SJS/TEN in Thai population. PLoS ONE. 2014;9:e94294.
Dong D, Sung C, Finkelstein EA. Cost-effectiveness of HLA-B*1502 genotyping in adult patients with newly diagnosed epilepsy in Singapore. Neurology. 2012;79:1259–67.
Rens NE, Uyl-de Groot CA, Goldhaber-Fiebert JD, Croda J, Andrews JR. Cost-effectiveness of a pharmacogenomic test for stratified isoniazid dosing in treatment of active tuberculosis. Clin Infect Dis. 2020;71:3136–43.
Pruis SL, Jeon YK, Pearce F, Thong BY, Aziz MIA. Cost-effectiveness of sequential urate lowering therapies for the management of gout in Singapore. J Med Econ. 2020;23:838–47.
You JH, Tsui KK, Wong RS, Cheng G. Cost-effectiveness of dabigatran versus genotype-guided management of warfarin therapy for stroke prevention in patients with atrial fibrillation. PLoS ONE. 2012;7:e39640.
Park DJ, Kang JH, Lee JW, Lee KE, Wen L, Kim TJ, et al. Cost-effectiveness analysis of HLA-B5801 genotyping in the treatment of gout patients with chronic renal insufficiency in Korea. Arthritis Care Res (Hoboken). 2015;67:280–7.
Oh KT, Anis AH, Bae SC. Pharmacoeconomic analysis of thiopurine methyltransferase polymorphism screening by polymerase chain reaction for treatment with azathioprine in Korea. Rheumatol (Oxf). 2004;43:156–63.
Chong HY, Lim YH, Prawjaeng J, Tassaneeyakul W, Mohamed Z, Chaiyakunapruk N. Cost-effectiveness analysis of HLA-B*58: 01 genetic testing before initiation of allopurinol therapy to prevent allopurinol-induced Stevens-Johnson syndrome/toxic epidermal necrolysis in a Malaysian population. Pharmacogenet Genomics. 2018;28:56–67.
Chong HY, Mohamed Z, Tan LL, Wu DBC, Shabaruddin FH, Dahlui M, et al. Is universal HLA-B*15:02 screening a cost-effective option in an ethnically diverse population? A case study of Malaysia. Br J Dermatol. 2017;177:1102–12.
Rattanavipapong W, Koopitakkajorn T, Praditsitthikorn N, Mahasirimongkol S, Teerawattananon Y. Economic evaluation of HLA-B*15:02 screening for carbamazepine-induced severe adverse drug reactions in Thailand. Epilepsia. 2013;54:1628–38.
Diamandis EP. Cancer biomarkers: can we turn recent failures into success? J Natl Cancer Inst. 2010;102:1462–7.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021. https://doi.org/10.3322/caac.21660.
Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global Burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76:2982–3021.
Berm EJ, Looff MD, Wilffert B, Boersma C, Annemans L, Vegter S, et al. Economic evaluations of pharmacogenetic and pharmacogenomic screening tests: a systematic review. Second update Lit PLoS ONE. 2016;11:e0146262.
Wong WB, Carlson JJ, Thariani R, Veenstra DL. Cost effectiveness of pharmacogenomics: a critical and systematic review. Pharmacoeconomics. 2010;28:1001–13.
Karamperis K, Koromina M, Papantoniou P, Skokou M, Kanellakis F, Mitropoulos K, et al. Economic evaluation in psychiatric pharmacogenomics: a systematic review. Pharmacogenomics J. 2021;21:533–41.
McKillip RP, Borden BA, Galecki P, Ham SA, Patrick-Miller L, Hall JP, et al. Patient perceptions of care as influenced by a large institutional pharmacogenomic implementation program. Clin Pharm Ther. 2017;102:106–14.
Planelles B, Margarit C, Inda MD, Ballester P, Muriel J, Barrachina J, et al. Gender based differences, pharmacogenetics and adverse events in chronic pain management. Pharmacogenomics J. 2020;20:320–8.
Acknowledgements
AS was supported by Universiti Teknologi MARA under grant no. 600-RMC/DINAMIK-POSTDOC 5/3 (010/2020). We would like to thank Nur Izzati Izni Rusli from Center for Diploma Studies, Universiti Tun Hussein Onn Malaysia and Siti Arifah Mohd Turjah from Faculty of Biotechnology and Biomolecular Sciences, Universiti Putra Malaysia for their kind help in data management. CM participation was enabled by funding from the European Developing Countries Clinical Trial Partnerships (EDCTP) grant TMA2016SF.
Author information
Authors and Affiliations
Contributions
Conceptualization, LKT and MZS; methodology, AS; validation, LKT, MZS, and CM; formal analysis, AS; data curation, AS; writing—original draft preparation, AS; writing—review and editing, LKT, MZS, and CM; supervision, LKT and MZS. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sukri, A., Salleh, M.Z., Masimirembwa, C. et al. A systematic review on the cost effectiveness of pharmacogenomics in developing countries: implementation challenges. Pharmacogenomics J 22, 147–159 (2022). https://doi.org/10.1038/s41397-022-00272-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41397-022-00272-w