Chinese Journal of Organic Chemistry ›› 2021, Vol. 41 ›› Issue (10): 4050-4058.DOI: 10.6023/cjoc202105044 Previous Articles Next Articles
Special Issue: 南开大学化学学科创立100周年; 热点论文虚拟合集
ARTICLES
收稿日期:2021-05-25
修回日期:2021-06-22
发布日期:2021-07-05
通讯作者:
王浩, 陈弓
基金资助:
Fangfang Song, Shiyang Zhu, Hao Wang( ), Gong Chen(
), Gong Chen( )
)
Received:2021-05-25
Revised:2021-06-22
Published:2021-07-05
Contact:
Hao Wang, Gong Chen
Supported by:Share
Fangfang Song, Shiyang Zhu, Hao Wang, Gong Chen. Iridium-Catalyzed Intermolecular N—N Coupling for Hydrazide Synthesis Using N-Benzoyloxycarbamates as Acyl Nitrene Precursor[J]. Chinese Journal of Organic Chemistry, 2021, 41(10): 4050-4058.
| Entry | Variation from the standard conditions | Yielda/% |
|---|---|---|
| 1 | Standard condition | 97 (94)b |
| 2 | No [Cp*IrCl2]2 | No reaction |
| 3 | Solvent free | 92 |
| 4 | [1]=0.1 mol/L | 95 |
| 5 | 1.2 equiv. 2e was added | 82 |
| 6 | HFIP as solvent | No reaction |
| 7 | DCE as solvent | 93 |
| 8 | Ar atmosphere | 92 |
| 9 | FeCl2•4H2O (5 mol%) as catalyst | No reaction |
| 10 | T=60 ℃ | 77 |
| 11 | AgSbF6 (5 mol%) | 15 |
| Entry | Variation from the standard conditions | Yielda/% |
|---|---|---|
| 1 | Standard condition | 97 (94)b |
| 2 | No [Cp*IrCl2]2 | No reaction |
| 3 | Solvent free | 92 |
| 4 | [1]=0.1 mol/L | 95 |
| 5 | 1.2 equiv. 2e was added | 82 |
| 6 | HFIP as solvent | No reaction |
| 7 | DCE as solvent | 93 |
| 8 | Ar atmosphere | 92 |
| 9 | FeCl2•4H2O (5 mol%) as catalyst | No reaction |
| 10 | T=60 ℃ | 77 |
| 11 | AgSbF6 (5 mol%) | 15 |
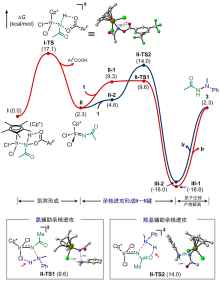

| [1] |
(a) Ke, S.; Sun, T.; Liang, Y.; Yang, Z. Chin. J. Org. Chem. 2010, 30, 1820. (in Chinese)
|
|
(柯少勇, 孙婷婷, 梁英, 杨自文, 有机化学, 2010, 30, 1820.)
|
|
|
(b) Blair, L. M.; Sperry, J. J. Nat. Prod. 2013, 76, 794.
doi: 10.1021/np400124n |
|
|
(c) Chen, L.; Deng, Z.; Zhao, C. ACS Chem. Biol. 2021, 16, 559.
doi: 10.1021/acschembio.1c00052 |
|
| [2] |
(a) Okuhara, M.; Kuroda, Y.; Goto, T.; Okamoto, M.; Terano, H.; Kohsaka, M.; Aoki, H.; Imanaka, H. J. Antibiot. 1980, 33, 13.
pmid: 16441076 |
|
(b) Ogita, T.; Gunji, S.; Fukazawa, Y.; Terahara, A.; Kinoshita, T.; Nagaki, H.; Beppu, T. Tetrahedron Lett. 1983, 24, 2283.
doi: 10.1016/S0040-4039(00)81904-8 pmid: 16441076 |
|
|
(c) Broberg, A.; Menkis, A.; Vasiliauskas, R. J. Nat. Prod. 2006, 69, 97.
pmid: 16441076 |
|
|
(d) Wang, K.-K. A.; Ng, T. L.; Wang, P.; Huang, Z.; Balskus, E. P.; van der Donk, W. A. Nat. Commun. 2018, 9, 3687.
doi: 10.1038/s41467-018-06083-7 pmid: 16441076 |
|
| [3] |
Forquet, V.; Sabaté, C. M.; Jacob, G.; Guelou, Y.; Delalu, H.; Darwich, C. Chem.-Asian J. 2015, 10, 1668.
doi: 10.1002/asia.v10.8 |
| [4] |
Diccianni, J. B.; Hu, C.; Diao, T. Angew. Chem.,Int. Ed. 2016, 55, 7534.
doi: 10.1002/anie.v55.26 |
| [5] |
(a) Ragnarsson, U. Chem. Soc. Rev. 2001, 30, 205.
doi: 10.1039/b010091a pmid: 11700143 |
|
(b) Wolter, M.; Klapars, A.; Buchwald, S. L. Org. Lett. 2001, 3, 3803.
pmid: 11700143 |
|
| [6] |
(a) Evans, D. A.; Johnson, D. S. Org. Lett. 1999, 1, 595.
pmid: 10823188 |
|
(b) Feng, G.; Wang, X.; Jin, J. Eur. J. Org. Chem. 2019, 6728.
pmid: 10823188 |
|
|
(c) Chen, J.; Shen, X.; Lu, Z. J. Am. Chem. Soc. 2020, 142, 14455.
doi: 10.1021/jacs.0c07258 pmid: 10823188 |
|
| [7] |
Guo, Q.; Lu, Z. Synthesis 2017, 49, 3835.
doi: 10.1055/s-0036-1588512 |
| [8] |
(a) Rosen, B. R.; Werner, E. W.; O'Brien, A. G.; Baran, P. S. J. Am. Chem. Soc. 2014, 136, 5571.
doi: 10.1021/ja5013323 pmid: 33974802 |
|
(b) Pandit, P.; Yamamoto, K.; Nakamura, T.; Nishimura, K.; Kurashige, Y.; Yanai, T.; Nakamura, G.; Masaoka, S.; Furukawa, K.; Yakiyama, Y.; Kawano, M.; Higashibayashi, S. Chem. Sci. 2015, 6, 4160.
doi: 10.1039/C5SC00946D pmid: 33974802 |
|
|
(c) Ryan, M. C.; Martinelli, J. R.; Stahl, S. S. J. Am. Chem. Soc. 2018, 140, 9074.
doi: 10.1021/jacs.8b05245 pmid: 33974802 |
|
|
(d) Feng, N.; Hou, Z.; Xu, H. Chin. J. Org. Chem. 2019, 39, 1424. (in Chinese)
doi: 10.6023/cjoc201812007 pmid: 33974802 |
|
|
(冯恩祺, 侯中伟, 徐海超, 有机化学, 2019, 39, 1424.)
doi: 10.6023/cjoc201812007 pmid: 33974802 |
|
|
(e) Yin, D.; Jin, J. Eur. J. Org. Chem. 2019, 2019, 5646.
doi: 10.1002/ejoc.201900763 pmid: 33974802 |
|
|
(f) Ryan, M. C.; Kim, Y. J.; Gerken, J. B.; Wang, F.; Aristov, M. M.; Martinelli, J. R.; Stahl, S. S. Chem. Sci. 2020, 11, 1170.
doi: 10.1039/C9SC04305E pmid: 33974802 |
|
|
(g) Vemuri, P. Y.; Patureau, F. W. Org. Lett. 2021, 23, 3902.
doi: 10.1021/acs.orglett.1c01034 pmid: 33974802 |
|
| [9] |
(a) Wallace, R. G. Aldrichim. Acta 1980, 13, 3.
|
|
(b) Vidal, J.; Hannachi, J. C.; Hourdin, G.; Mulatier, J. C.; Collet, A. Tetrahedron Lett. 1998, 39, 8845.
doi: 10.1016/S0040-4039(98)01983-2 |
|
|
(c) Kang, C. W.; Sarnowski, M. P.; Elbatrawi, Y. M.; Del Valle, J. R. J. Org. Chem. 2017, 82, 1833.
doi: 10.1021/acs.joc.6b02718 |
|
|
(d) Lei, L.; Li, C.; Mo, D. Chin. J. Org. Chem. 2019, 39, 2989. (in Chinese)
doi: 10.6023/cjoc201904037 |
|
|
(雷禄, 李承璟, 莫冬亮, 有机化学, 2019, 39, 2989.)
doi: 10.6023/cjoc201904037 |
|
|
(e) Zhang, Y.; He, J.; Song, P.; Wang, Y.; Zhu, S. CCS Chem. 2020, 2, 2259.
|
|
| [10] |
(a) Li, J.; Cisar, J. S.; Zhou, C.-Y.; Vera, B.; Williams, H.; Rodríguez, A. D.; Cravatt, B. F.; Romo, D. Nat. Chem. 2013, 5, 510.
doi: 10.1038/nchem.1653 |
|
(b) Kono, M.; Harada, S.; Nemoto, T. Chem.-Eur. J. 2019, 25, 3119.
|
|
|
(c) Maestre, L.; Dorel, R.; Pablo, O.; Escofet, I.; Sameera, W. M. C.; Alvarez, E.; Maseras, F.; Diaz-Requejo, M. M.; Echavarren, A. M.; Perez, P. J. J. Am. Chem. Soc. 2017, 139, 2216.
doi: 10.1021/jacs.6b08219 |
|
|
(d) Dehghany, M.; Eshon, J.; Roberts, J. M.; Schomaker, J. M. In Silver Catalysis in Organic Synthesis, Vol. 4, Eds.: Li, C.-J.; Bi, X., John Wiley & Sons, Weinheim, 2019, Chapter 8, pp. 439-532.
|
|
| [11] |
(a) Shimbayashi, T.; Sasakura, K.; Eguchi, A.; Okamoto, K.; Ohe, K. Chem.-Eur. J. 2019, 25, 3156.
doi: 10.1002/chem.201803716 pmid: 30183111 |
|
(b) van Vliet, K. M.; de Bruin, B. ACS Catal. 2020, 10, 4751.
doi: 10.1021/acscatal.0c00961 pmid: 30183111 |
|
|
(c) Wang, Y.-C.; Lai, X.-J.; Huang, K.; Yadav, S.; Qiu, G.; Zhang, L.; Zhou, H. Org. Chem. Front. 2021, 8, 1677.
doi: 10.1039/D0QO01360A pmid: 30183111 |
|
|
(d) Hong, S. Y.; Hwang, Y.; Lee, M.; Chang, S. Acc. Chem. Res. 2021, 54, 2683.
doi: 10.1021/acs.accounts.1c00198 pmid: 30183111 |
|
|
(e) Wu, L.-Y.; Zhong, D.; Liu, W.-B. Chin. J. Org. Chem. 2021, 41, 4083. (in Chinese)
pmid: 30183111 |
|
|
(邬林洋, 钟大猷, 刘文博, 有机化学, 2021, 41, 4083.)
pmid: 30183111 |
|
| [12] |
(a) Ng, K.-H.; Chan, A. S. C.; Yu, W.-Y. J. Am. Chem. Soc. 2010, 132, 12862.
doi: 10.1021/ja106364r pmid: 30978019 |
|
(b) Grohmann, C.; Wang, H.; Glorius, F. Org. Lett. 2013, 15, 3014.
doi: 10.1021/ol401209f pmid: 30978019 |
|
|
(c) John, A.; Byun, J.; Nicholas, K. M. Chem. Commun. 2013, 49, 10965.
doi: 10.1039/c3cc46412a pmid: 30978019 |
|
|
(d) Zhou, B.; Du, J.; Yang, Y.; Feng, H.; Li, Y. Org. Lett. 2014, 16, 592.
doi: 10.1021/ol403477w pmid: 30978019 |
|
|
(e) Shi, J.; Zhao, G.; Wang, X.; Xu, H. E.; Yi, W. Org. Biomol. Chem. 2014, 12, 6831.
doi: 10.1039/C4OB00637B pmid: 30978019 |
|
|
(f) Huh, S.; Hong, S. Y.; Chang, S. Org. Lett. 2019, 21, 2808.
doi: 10.1021/acs.orglett.9b00791 pmid: 30978019 |
|
|
(g) Wang, H.; Park, Y.; Bai, Z. Q.; Chang, S.; He, G.; Chen, G. J. Am. Chem. Soc. 2019, 141, 7194.
doi: 10.1021/jacs.9b02811 pmid: 30978019 |
|
|
(h) Guo, Q.; Ren, X.; Lu, Z. Org. Lett. 2019, 21, 880.
doi: 10.1021/acs.orglett.8b03299 pmid: 30978019 |
|
|
(i) Jung, H.; Keum, H.; Kweon, J.; Chang, S. J. Am. Chem. Soc. 2020, 142, 5811.
doi: 10.1021/jacs.0c00868 pmid: 30978019 |
|
| [13] |
(a) Bräse, S.; Gil, C.; Knepper, K.; Zimmermann, V. Angew. Chem., nt. Ed. 2005, 44, 5188.
pmid: 32357006 |
|
(b) Clayden, J.; Donnard, M.; Lefranc, J.; Tetlow, D. J. Chem. Commun. 2011, 47, 4624.
doi: 10.1039/c1cc00049g pmid: 32357006 |
|
|
(c) Zhou, H.; Hong, J.; Huang, J.; Chen, Z. Asian J. Org. Chem. 2017, 6, 817.
doi: 10.1002/ajoc.v6.7 pmid: 32357006 |
|
|
(d) Li, W. H.; Dong, L. Adv. Synth. Catal. 2018, 360, 1104.
doi: 10.1002/adsc.v360.6 pmid: 32357006 |
|
|
(e) Bakhoda, A.; Jiang, Q.; Badiei, Y. M.; Bertke, J. A.; Cundari, T. R.; Warren, T. H. Angew. Chem., nt. Ed. 2019, 58, 3421.
pmid: 32357006 |
|
|
(f) Yoshitake, M.; Hayashi, H.; Uchida, T. Org. Lett. 2020, 22, 4021.
doi: 10.1021/acs.orglett.0c01373 pmid: 32357006 |
|
| [14] |
Wang, H.; Jung, H.; Song, F.; Zhu, S.; Bai, Z.; Chen, D.; He, G.; Chang, S.; Chen, G. Nat. Chem. 2021, 13, 378.
doi: 10.1038/s41557-021-00650-0 |
| [15] |
Ball, R. G.; Graham, W. A. G.; Heinekey, D. M.; Hoyano, J. K.; McMaster, A. D.; Mattson, B. M.; Michel, S. T. Inorg. Chem. 1990, 29, 2023.
doi: 10.1021/ic00335a051 |
| [16] |
Le Grel, P.; Salaün, A.; Mocquet, C.; Le Grel, B.; Roisnel, T.; Potel, M. J. Org. Chem. 2011, 76, 8756.
doi: 10.1021/jo201390b |
| [17] |
Han, S.; Shin, Y.; Sharma, S.; Mishra, N. K.; Park, J.; Kim, M.; Kim, M.; Jang, J.; Kim, I. S. Org. Lett. 2014, 16, 2494.
doi: 10.1021/ol500865j |
| [18] |
Zhan, F.; Liang, G. Angew. Chem., nt. Ed. 2013, 52, 1266.
|
| [1] | Wang Jiang, Zhuangzhi Shi. Recent Progress in meta-/para-Selective Aromatic C—H Borylation [J]. Chinese Journal of Organic Chemistry, 2023, 43(5): 1691-1705. |
| [2] | Xing Yang, Xu Liu, Lijia Wang. Recent Progress in Transition Metal Catalyzed C(sp3)—H Nitrene Insertion Reactions Assisted by Directing Groups [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 914-923. |
| [3] | Huan Xu, Hongfei Wu, Xiaoming Zhang, Xingxing Lu, Tengda Sun, Yue Qi, Yufan Lin, Xinling Yang, Li Zhang, Yun Ling. Design, Synthesis and Bioactivity of Sulfonyl Hydrazides and Hydrazides Containing Fragment 1,2,3,4-Tetrahydroisoquinoline [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 725-733. |
| [4] | Zhixia Jing, Jianxi Du, Ping Jiang, Keyume Ablajan. Tetrabutylammonium Iodide-Mediated One-Pot Construction of 1,3,4-Oxadiazole Derivatives with Alkyl Amide and Hydrazine [J]. Chinese Journal of Organic Chemistry, 2023, 43(11): 3930-3938. |
| [5] | Kemiao Hong, Jingjing Huang, Minghan Yao, Xinfang Xu. Recent Advances in Nitrene/Alkyne Metathesis Cascade Reaction [J]. Chinese Journal of Organic Chemistry, 2022, 42(2): 344-352. |
| [6] | Haojie Ma, Xiaoqiang Zhou, Bo Han, Ran Li, Xueyan Hou, Xingyue Ji, Yuqi Zhang, Guosheng Huang, Jijiang Wang. A Catalyst-Free One-Pot Protocol for the Construction of Substituted Sulfonyl Pyrazoles [J]. Chinese Journal of Organic Chemistry, 2021, 41(9): 3710-3716. |
| [7] | Jin Zhou, Hongshun Sun, Yulong Li, Hong Jiang, Cheng Guo, Linjiang Shen. Synthesis and Relaxivity of One Macrocyclic Binuclear Nonionic Magnetic Resonance Contrast Agent [J]. Chinese Journal of Organic Chemistry, 2021, 41(7): 2767-2773. |
| [8] | Haojie Ma, Zhou Sun, Jinlei Liu, Xia Zhang, Huali Cui, Yuqi Zhang, Jijiang Wang. CBr4-Mediated Intermolecular Cyclization Reaction: Efficient Synthesis of Substituted N-Acylpyrazoles [J]. Chinese Journal of Organic Chemistry, 2021, 41(11): 4353-4360. |
| [9] | Linyang Wu, Dayou Zhong, Wenbo Liu. Ligand-Free Iron-Catalyzed Intramolecular Amination of C(sp3)—H Bond for the Synthesis of Imidazolinones [J]. Chinese Journal of Organic Chemistry, 2021, 41(10): 4083-4087. |
| [10] | Peng Mei, Zheng Yangfan, Huang Hao, Ye Jia, Deng Xingguo, He Chunlian. Visible-Light-Induced Cycloaddition Involving N-Propargylanilines with Arylsulfonylhydrazides: Rapid Access to 3-Sulfonated Quinoline Derivatives without Base and Catalyst [J]. Chinese Journal of Organic Chemistry, 2020, 40(7): 2078-2085. |
| [11] | Xu Weigang, Huang Danfeng, Wang Kehu, Zhao Fangxia, Zhao Zhuanxia, Hu Yongqing, Su Yingpeng, Hu Yulai. Study on the Hydrocyanation Reaction of Trifluoromethylated Acylhydrazones [J]. Chinese Journal of Organic Chemistry, 2020, 40(4): 922-929. |
| [12] | Wang Lin, Yang Lili, Ou Yunfu, Xu Shihai, Lin Qifu, Yang Dingqiao. Platinum-Catalyzed syn-Stereocontrolled Ring-Opening of Oxabicyclic Alkenes with Arylsulfonyl Hydrazides [J]. Chinese Journal of Organic Chemistry, 2020, 40(12): 4228-4236. |
| [13] | Liu Jiaxin, Huang Danfeng, Wang Xiaoping, Zong Wuzhong, Su Yingpeng, Wang Kehu, Hu Yulai. Tin Powder Promoted Synthesis of α-Trifluoromethyl Homoallylic Hydrazides [J]. Chin. J. Org. Chem., 2019, 39(6): 1767-1775. |
| [14] | Zhang Zhenlei, Qian Peng, Zha Zhenggen. Copper-Catalyzed Aerobic Oxidative Coupling of Aromatic Sulfonyl Hydrazides with Amines:A New Access to Aromatic Sulfonamides [J]. Chin. J. Org. Chem., 2019, 39(5): 1316-1322. |
| [15] | Zhu Fuyuan, Wang Yanmei, He Mingchuang, Yan Zhaohua, Lin Sen. Tetrabutylammonium Iodide Promoted Thioetherification of Arylsulfonylhydrazides with 4-Hydroxycoumarins [J]. Chin. J. Org. Chem., 2019, 39(4): 1175-1180. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||