当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Keto‐Enol Tautomerism in Nucleobase‐Substituted Aldols
ChemistrySelect ( IF 2.1 ) Pub Date : 2018-12-07 , DOI: 10.1002/slct.201802538 Mariano J. Nigro 1 , Adolfo M. Iribarren 1 , Sergio L. Laurella 2 , Elizabeth S. Lewkowicz 1
ChemistrySelect ( IF 2.1 ) Pub Date : 2018-12-07 , DOI: 10.1002/slct.201802538 Mariano J. Nigro 1 , Adolfo M. Iribarren 1 , Sergio L. Laurella 2 , Elizabeth S. Lewkowicz 1
Affiliation
|
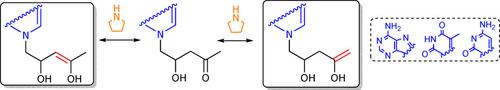
Acyclic nucleosides, which exhibit significant antiviral activity, are usually synthesised using traditional chemical strategies. However, the efficient and selective formation of carbon‐carbon bonds using small organic molecules as catalysts provides a promising alternative route for the sustainable synthesis of this family of compounds. Following this organocatalytic strategy, 5‐(adenyl, thyminyl and cytosyl)‐4‐hydroxy‐2‐pentanones were prepared by the pyrrolidine catalysed reaction between the 2‐oxoethyl derivative of the corresponding nucleobases and acetone. In order to investigate the keto‐enol equilibrium of these compounds in basic media, H‐D exchange studies were carried out by 1H and 13C NMR spectroscopy. The obtained results suggest that the mechanism by which this exchange occurs is of first order with respect to all the substrates, but of second order with regard to pyrrolidine in the case of the cytosine and adenine derivatives and of first order for the thymine analogue. Theoretical calculations of the structures involved in this equilibrium also suggest that the stability of the different ionic intermediates depends on the pKa of the corresponding nucleobases.
中文翻译:

核碱基取代的羟醛中的酮-烯醇互变异构
通常使用传统的化学策略合成具有显着抗病毒活性的无环核苷。但是,使用有机小分子作为催化剂有效而选择性地形成碳碳键,为该族化合物的可持续合成提供了一条有希望的替代途径。按照这种有机催化策略,通过吡咯烷催化相应核碱基的2-氧乙基衍生物与丙酮之间的反应,制备了5-(腺苷,胸腺嘧啶和胞嘧啶)-4-羟基-2-戊酮。为了研究这些化合物在碱性介质中的酮-烯醇平衡,我们用1 H和13进行了H-D交换研究。13 C NMR光谱。所获得的结果表明,这种交换发生的机理对于所有底物而言是一阶的,但是对于胞嘧啶和腺嘌呤衍生物而言,对于吡咯烷而言是二阶的,对于胸腺嘧啶类似物而言是一阶的。参与这一平衡结构的理论计算还表明,不同的离子中间体的稳定性取决于的pK一个相应的核碱基。
更新日期:2018-12-07
中文翻译:

核碱基取代的羟醛中的酮-烯醇互变异构
通常使用传统的化学策略合成具有显着抗病毒活性的无环核苷。但是,使用有机小分子作为催化剂有效而选择性地形成碳碳键,为该族化合物的可持续合成提供了一条有希望的替代途径。按照这种有机催化策略,通过吡咯烷催化相应核碱基的2-氧乙基衍生物与丙酮之间的反应,制备了5-(腺苷,胸腺嘧啶和胞嘧啶)-4-羟基-2-戊酮。为了研究这些化合物在碱性介质中的酮-烯醇平衡,我们用1 H和13进行了H-D交换研究。13 C NMR光谱。所获得的结果表明,这种交换发生的机理对于所有底物而言是一阶的,但是对于胞嘧啶和腺嘌呤衍生物而言,对于吡咯烷而言是二阶的,对于胸腺嘧啶类似物而言是一阶的。参与这一平衡结构的理论计算还表明,不同的离子中间体的稳定性取决于的pK一个相应的核碱基。



























 京公网安备 11010802027423号
京公网安备 11010802027423号