当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Macromolecular-clustered facial amphiphilic antimicrobials.
Nature Communications ( IF 16.6 ) Pub Date : 2018-12-07 , DOI: 10.1038/s41467-018-07651-7 Md Anisur Rahman 1 , Marpe Bam 2 , Edgar Luat 1 , Moumita Sharmin Jui 1 , Mitra S Ganewatta 1 , Tinom Shokfai 3 , Mitzi Nagarkatti 2 , Alan W Decho 3 , Chuanbing Tang 1
Nature Communications ( IF 16.6 ) Pub Date : 2018-12-07 , DOI: 10.1038/s41467-018-07651-7 Md Anisur Rahman 1 , Marpe Bam 2 , Edgar Luat 1 , Moumita Sharmin Jui 1 , Mitra S Ganewatta 1 , Tinom Shokfai 3 , Mitzi Nagarkatti 2 , Alan W Decho 3 , Chuanbing Tang 1
Affiliation
|
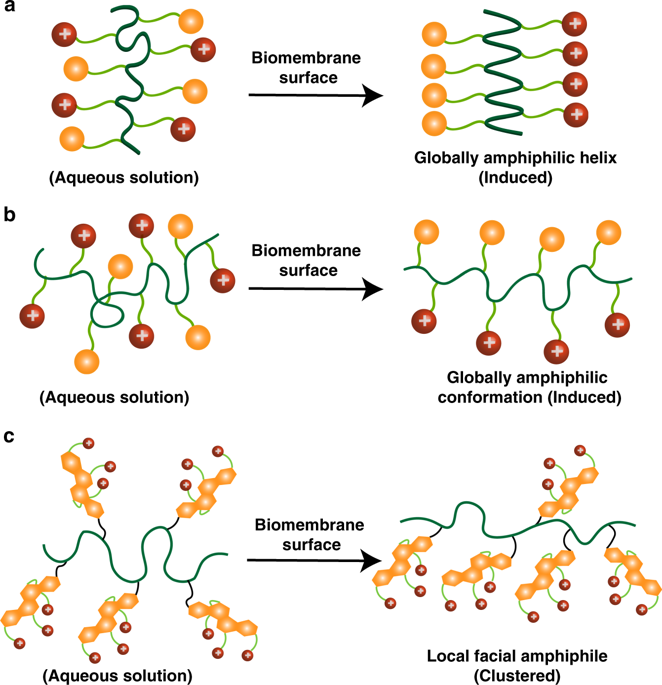
Bacterial infections and antibiotic resistance, particularly by Gram-negative pathogens, have become a global healthcare crisis. We report the design of a class of cationic antimicrobial polymers that cluster local facial amphiphilicity from repeating units to enhance interactions with bacterial membranes without requiring a globally conformational arrangement associated with highly unfavorable entropic loss. This concept of macromolecular architectures is demonstrated with a series of multicyclic natural product-based cationic polymers. We have shown that cholic acid derivatives with three charged head groups are more potent and selective than lithocholic and deoxycholic counterparts, particularly against Gram-negative bacteria. This is ascribed to the formation of true facial amphiphilicity with hydrophilic ion groups oriented on one face and hydrophobic multicyclic hydrocarbon structures on the opposite face. Such local facial amphiphilicity is clustered via a flexible macromolecular backbone in a concerted way when in contact with bacterial membranes.
中文翻译:

大分子簇面部两亲抗菌剂。
细菌感染和抗生素耐药性,特别是革兰氏阴性病原体引起的细菌感染和抗生素耐药性,已成为全球医疗保健危机。我们报告了一类阳离子抗菌聚合物的设计,该聚合物将重复单元的局部表面两亲性聚集在一起,以增强与细菌膜的相互作用,而不需要与高度不利的熵损失相关的全局构象排列。这种大分子结构的概念通过一系列基于多环天然产物的阳离子聚合物得到了证明。我们已经证明,具有三个带电头基的胆酸衍生物比石胆酸和脱氧胆酸对应物更有效和更具选择性,特别是针对革兰氏阴性细菌。这归因于形成真正的表面两亲性,亲水性离子基团位于一个面上,而疏水性多环烃结构位于相反的面上。当与细菌膜接触时,这种局部表面两亲性通过柔性大分子主链以协调的方式聚集。
更新日期:2018-12-07
中文翻译:

大分子簇面部两亲抗菌剂。
细菌感染和抗生素耐药性,特别是革兰氏阴性病原体引起的细菌感染和抗生素耐药性,已成为全球医疗保健危机。我们报告了一类阳离子抗菌聚合物的设计,该聚合物将重复单元的局部表面两亲性聚集在一起,以增强与细菌膜的相互作用,而不需要与高度不利的熵损失相关的全局构象排列。这种大分子结构的概念通过一系列基于多环天然产物的阳离子聚合物得到了证明。我们已经证明,具有三个带电头基的胆酸衍生物比石胆酸和脱氧胆酸对应物更有效和更具选择性,特别是针对革兰氏阴性细菌。这归因于形成真正的表面两亲性,亲水性离子基团位于一个面上,而疏水性多环烃结构位于相反的面上。当与细菌膜接触时,这种局部表面两亲性通过柔性大分子主链以协调的方式聚集。


























 京公网安备 11010802027423号
京公网安备 11010802027423号