当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Insight into the Thermophilic Mechanism of a Glycoside Hydrolase Family 5 β-Mannanase
Journal of Agricultural and Food Chemistry ( IF 6.1 ) Pub Date : 2018-12-06 00:00:00 , DOI: 10.1021/acs.jafc.8b04860 Weina Liu 1 , Tao Tu 1 , Yuan Gu 1 , Yuan Wang 1 , Fei Zheng 1 , Jie Zheng 1 , Yaru Wang 1 , Xiaoyun Su 1 , Bin Yao 1 , Huiying Luo 1
Journal of Agricultural and Food Chemistry ( IF 6.1 ) Pub Date : 2018-12-06 00:00:00 , DOI: 10.1021/acs.jafc.8b04860 Weina Liu 1 , Tao Tu 1 , Yuan Gu 1 , Yuan Wang 1 , Fei Zheng 1 , Jie Zheng 1 , Yaru Wang 1 , Xiaoyun Su 1 , Bin Yao 1 , Huiying Luo 1
Affiliation
|
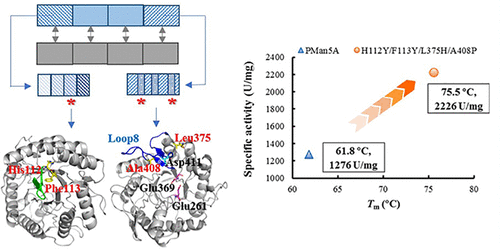
To study the molecular basis for thermophilic β-mannanase of glycoside hydrolase family 5, two β-mannanases, TlMan5A and PMan5A, from Talaromyces leycettanus JCM12802 and Penicillium sp. WN1 were used as models. The four residues, His112 and Phe113, located near the antiparallel β-sheet at the barrel bottom and Leu375 and Ala408 from loop 7 and loop 8 of PMan5A, were inferred to be key thermostability contributors through module substitution, truncation, and site-directed mutagenesis. The effects of these four residues on the thermal properties followed the order H112Y > A408P > L375H > F113Y and were strongly synergetic. These results were interpreted structurally using molecular dynamics (MD) simulations, which showed that improved hydrophobic interactions in the inner wall of the β-barrel and the rigidity of loop 8 were caused by the outside domain of the barrel bottom and proline, respectively. The TIM barrel bottom and four specific residues responsible for the thermostability of GH5 β-mannanases were elucidated.
中文翻译:

对糖苷水解酶家族5β-甘露聚糖酶的嗜热机理的认识。
为了研究糖苷水解酶家族5的嗜热β-甘露聚糖酶的分子基础,得自Talaromyces leycettanus JCM12802和Penicillium sp。的两种β-甘露聚糖酶,T1 Man5A和P Man5A 。WN1被用作模型。位于桶底部反平行β-折叠附近的四个残基His112和Phe113,以及P的第7环和第8环的Leu375和Ala408通过模块替换,截短和定点诱变,推断Man5A是关键的热稳定性贡献者。这四个残基对热性能的影响遵循H112Y> A408P> L375H> F113Y的顺序,并且具有很强的协同作用。使用分子动力学(MD)模拟从结构上解释了这些结果,结果表明,β-桶内壁中疏水性相互作用的改善和环8的刚度分别是由桶底部和脯氨酸的外域引起的。阐明了TIM桶底部和负责GH5β-甘露聚糖酶热稳定性的四个特定残基。
更新日期:2018-12-06
中文翻译:

对糖苷水解酶家族5β-甘露聚糖酶的嗜热机理的认识。
为了研究糖苷水解酶家族5的嗜热β-甘露聚糖酶的分子基础,得自Talaromyces leycettanus JCM12802和Penicillium sp。的两种β-甘露聚糖酶,T1 Man5A和P Man5A 。WN1被用作模型。位于桶底部反平行β-折叠附近的四个残基His112和Phe113,以及P的第7环和第8环的Leu375和Ala408通过模块替换,截短和定点诱变,推断Man5A是关键的热稳定性贡献者。这四个残基对热性能的影响遵循H112Y> A408P> L375H> F113Y的顺序,并且具有很强的协同作用。使用分子动力学(MD)模拟从结构上解释了这些结果,结果表明,β-桶内壁中疏水性相互作用的改善和环8的刚度分别是由桶底部和脯氨酸的外域引起的。阐明了TIM桶底部和负责GH5β-甘露聚糖酶热稳定性的四个特定残基。



























 京公网安备 11010802027423号
京公网安备 11010802027423号