当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Pyrazoline-based colorimetric and fluorescent probe for detection of sulphite†
New Journal of Chemistry ( IF 3.3 ) Pub Date : 2018-11-30 00:00:00 , DOI: 10.1039/c8nj05017a Tomasz Uchacz 1, 2, 3, 4 , Gabriela Jajko 1, 2, 3, 4 , Andrzej Danel 4, 5, 6, 7, 8 , Paweł Szlachcic 4, 5, 6, 7, 8 , Szczepan Zapotoczny 1, 2, 3, 4
New Journal of Chemistry ( IF 3.3 ) Pub Date : 2018-11-30 00:00:00 , DOI: 10.1039/c8nj05017a Tomasz Uchacz 1, 2, 3, 4 , Gabriela Jajko 1, 2, 3, 4 , Andrzej Danel 4, 5, 6, 7, 8 , Paweł Szlachcic 4, 5, 6, 7, 8 , Szczepan Zapotoczny 1, 2, 3, 4
Affiliation
|
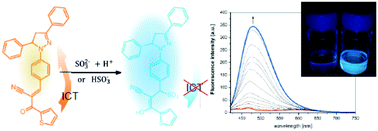
Two pyrazoline-based fluorescent and colorimetric probes have been synthesized and their photophysical properties have been investigated by means of electronic absorption and emission spectroscopy. The compounds differ from each other by the presence of a phenyl or thiophene end group attached to the α,β-unsaturated ketone. The probes detect sulphite anions in the Michael addition reaction to the α,β-unsaturated ketone with a high selectivity and good sensitivity (7.56 μM for phenyl and 4.87 μM for the thiophene counterpart). Here, the optical response is based on the recovery of triphenylpyrazoline fluorescence, which is largely blue-shifted as compared to weak charge transfer emission of the sensors. This large hypsochromic shift of the emission maximum along with a strong fluorescence enhancement (up to Isulphite/I0 = 43) can be an advantage in terms of the accurate evaluation of the fluorescence intensity ratio. The pyrazoline-based sensor decorated with thiophene is able to detect sulphite species in river water solutions with a good selectivity and sensitivity.
中文翻译:

基于吡唑啉的比色和荧光探针,用于检测亚硫酸盐†
合成了两种基于吡唑啉的荧光探针和比色探针,并通过电子吸收和发射光谱研究了它们的光物理性质。这些化合物彼此不同之处在于存在与α,β-不饱和酮相连的苯基或噻吩端基。探针以高选择性和良好的灵敏度(对苯基为7.56μM,对噻吩为4.87μM)检测到与α,β-不饱和酮的迈克尔加成反应中的亚硫酸根阴离子。在此,光学响应基于三苯基吡唑啉荧光的恢复,与传感器的弱电荷转移发射相比,三苯基吡唑啉荧光发生了蓝移。发射最大值的这种大的变色位移以及强烈的荧光增强(高达I就准确评估荧光强度比而言,亚硫酸盐/ I 0 = 43)可能是一个优势。用噻吩修饰的基于吡唑啉的传感器能够以良好的选择性和灵敏度检测河流水溶液中的亚硫酸盐物种。
更新日期:2018-11-30
中文翻译:

基于吡唑啉的比色和荧光探针,用于检测亚硫酸盐†
合成了两种基于吡唑啉的荧光探针和比色探针,并通过电子吸收和发射光谱研究了它们的光物理性质。这些化合物彼此不同之处在于存在与α,β-不饱和酮相连的苯基或噻吩端基。探针以高选择性和良好的灵敏度(对苯基为7.56μM,对噻吩为4.87μM)检测到与α,β-不饱和酮的迈克尔加成反应中的亚硫酸根阴离子。在此,光学响应基于三苯基吡唑啉荧光的恢复,与传感器的弱电荷转移发射相比,三苯基吡唑啉荧光发生了蓝移。发射最大值的这种大的变色位移以及强烈的荧光增强(高达I就准确评估荧光强度比而言,亚硫酸盐/ I 0 = 43)可能是一个优势。用噻吩修饰的基于吡唑啉的传感器能够以良好的选择性和灵敏度检测河流水溶液中的亚硫酸盐物种。



























 京公网安备 11010802027423号
京公网安备 11010802027423号