当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Improvement of the Activity and Stability of Starch-Debranching Pullulanase from Bacillus naganoensis via Tailoring of the Active Sites Lining the Catalytic Pocket
Journal of Agricultural and Food Chemistry ( IF 6.1 ) Pub Date : 2018-11-30 00:00:00 , DOI: 10.1021/acs.jafc.8b06002 Xinye Wang 1 , Yao Nie 1, 2 , Yan Xu 1, 2
Journal of Agricultural and Food Chemistry ( IF 6.1 ) Pub Date : 2018-11-30 00:00:00 , DOI: 10.1021/acs.jafc.8b06002 Xinye Wang 1 , Yao Nie 1, 2 , Yan Xu 1, 2
Affiliation
|
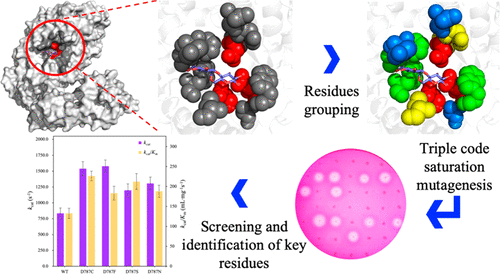
Pullulanases are well-known debranching enzymes that hydrolyze α-1,6-glycosidic linkages in starch and oligosaccharides. However, most of the pullulanases exhibit limited activity for practical applications. Here, two sites (787 and 621) lining the catalytic pocket of Bacillus naganoensis pullulanase were identified as being critical for enzymatic activity by triple-code saturation mutagenesis. Subsequently, both sites were subjected to NNK-based saturation mutagenesis to obtain positive variants. Among the variants showing enhanced activity, the enzymatic activity and specific activity of D787C were 1.5-fold higher than those of the wild-type (WT). D787C also showed a 1.8-fold increase in kcat and a 1.7-fold increase in kcat/Km. In addition, D787C maintained higher activity compared with that of WT at temperatures over 60 °C. All the positive variants showed higher acid resistance, with D787C maintaining 90% residual activity at pH 4.0. Thus, enzymes with improved properties were obtained by saturation mutagenesis at the active site.
中文翻译:

通过调整活性口袋衬里的活性位点,改善长野芽孢杆菌淀粉解支链淀粉酶的活性和稳定性
支链淀粉酶是众所周知的脱支酶,其水解淀粉和寡糖中的α-1,6-糖苷键。但是,大多数支链淀粉酶在实际应用中显示出有限的活性。在这里,通过三密码子饱和诱变,长条芽孢杆菌支链淀粉酶的催化口袋内衬的两个位点(787和621)被确定对酶活性至关重要。随后,对两个位点都进行了基于NNK的饱和诱变,以获得阳性变体。在表现出增强活性的变体中,D787C的酶促活性和比活性比野生型(WT)高1.5倍。D787C也显示在一个增加1.8倍ķ猫,并在1.7倍的增加ķ猫/ķ米。另外,在超过60°C的温度下,D787C与WT相比仍保持较高的活性。所有阳性变体均显示出较高的耐酸性,D787C在pH 4.0下保持90%的残留活性。因此,通过在活性位点进行饱和诱变获得了具有改善的特性的酶。
更新日期:2018-11-30
中文翻译:

通过调整活性口袋衬里的活性位点,改善长野芽孢杆菌淀粉解支链淀粉酶的活性和稳定性
支链淀粉酶是众所周知的脱支酶,其水解淀粉和寡糖中的α-1,6-糖苷键。但是,大多数支链淀粉酶在实际应用中显示出有限的活性。在这里,通过三密码子饱和诱变,长条芽孢杆菌支链淀粉酶的催化口袋内衬的两个位点(787和621)被确定对酶活性至关重要。随后,对两个位点都进行了基于NNK的饱和诱变,以获得阳性变体。在表现出增强活性的变体中,D787C的酶促活性和比活性比野生型(WT)高1.5倍。D787C也显示在一个增加1.8倍ķ猫,并在1.7倍的增加ķ猫/ķ米。另外,在超过60°C的温度下,D787C与WT相比仍保持较高的活性。所有阳性变体均显示出较高的耐酸性,D787C在pH 4.0下保持90%的残留活性。因此,通过在活性位点进行饱和诱变获得了具有改善的特性的酶。



























 京公网安备 11010802027423号
京公网安备 11010802027423号