当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Artificial chimeric exosomes for anti-phagocytosis and targeted cancer therapy†
Chemical Science ( IF 8.4 ) Pub Date : 2018-11-27 00:00:00 , DOI: 10.1039/c8sc03224f Kai-Long Zhang 1 , Ying-Jie Wang 1 , Jin Sun 2, 3 , Jie Zhou 1 , Chao Xing 1 , Guoming Huang 3 , Juan Li 1, 2 , Huanghao Yang 1
Chemical Science ( IF 8.4 ) Pub Date : 2018-11-27 00:00:00 , DOI: 10.1039/c8sc03224f Kai-Long Zhang 1 , Ying-Jie Wang 1 , Jin Sun 2, 3 , Jie Zhou 1 , Chao Xing 1 , Guoming Huang 3 , Juan Li 1, 2 , Huanghao Yang 1
Affiliation
|
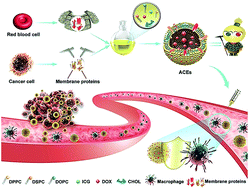
Development of exosome-based delivery systems is still facing some formidable challenges, including the lack of standardized isolation and purification methods, non-large-scale production and low drug-loading efficiency. Inspired by biomimetic technologies, we turned to the design of artificial chimeric exosomes (ACEs) constructed by integrating cell membrane proteins from multiple cell types into synthetic phospholipid bilayers. For benchmarking, hybrid membrane proteins derived from red blood cells (RBCs) and MCF-7 cancer cells were selected as models. The resulting ACEs were engineered much like “Emperor Qin's Terra-Cotta Warriors”, simultaneously equipped with armor (anti-phagocytosis capability from RBCs) and dagger-axes (homologous targeting ability from cancer cells). ACEs demonstrated higher tumor accumulation, lower interception and better antitumor therapeutic effect than plain liposomes in vivo, alongside large-scale standardized preparation, stable structure, high drug-loading capacity and custom-tailored functionality, highlighting the suitability of ACEs as promising alternatives of exosomes in clinical applications.
中文翻译:

用于抗吞噬作用和靶向癌症治疗的人工嵌合外泌体†
基于外泌体的递送系统的开发仍然面临一些艰巨的挑战,包括缺乏标准化的分离和纯化方法、非大规模生产和载药效率低等。受仿生技术的启发,我们转向设计人工嵌合外泌体(ACE),通过将多种细胞类型的细胞膜蛋白整合到合成磷脂双层中来构建。为了进行基准测试,选择源自红细胞 (RBC) 和 MCF-7 癌细胞的混合膜蛋白作为模型。由此产生的 ACE 的设计很像“秦始皇的兵马俑”,同时配备了盔甲(红细胞的抗吞噬能力)和匕首(癌细胞的同源靶向能力)。ACEs在体内表现出比普通脂质体更高的肿瘤积累、更低的拦截和更好的抗肿瘤治疗效果,同时大规模标准化制备、稳定的结构、高载药量和定制的功能,凸显了ACEs作为外泌体的有希望的替代品的适用性在临床应用中。
更新日期:2018-11-27
中文翻译:

用于抗吞噬作用和靶向癌症治疗的人工嵌合外泌体†
基于外泌体的递送系统的开发仍然面临一些艰巨的挑战,包括缺乏标准化的分离和纯化方法、非大规模生产和载药效率低等。受仿生技术的启发,我们转向设计人工嵌合外泌体(ACE),通过将多种细胞类型的细胞膜蛋白整合到合成磷脂双层中来构建。为了进行基准测试,选择源自红细胞 (RBC) 和 MCF-7 癌细胞的混合膜蛋白作为模型。由此产生的 ACE 的设计很像“秦始皇的兵马俑”,同时配备了盔甲(红细胞的抗吞噬能力)和匕首(癌细胞的同源靶向能力)。ACEs在体内表现出比普通脂质体更高的肿瘤积累、更低的拦截和更好的抗肿瘤治疗效果,同时大规模标准化制备、稳定的结构、高载药量和定制的功能,凸显了ACEs作为外泌体的有希望的替代品的适用性在临床应用中。



























 京公网安备 11010802027423号
京公网安备 11010802027423号