当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Golgi-targeting fluorescent probe for labile Fe(ii) to reveal an abnormal cellular iron distribution induced by dysfunction of VPS35†
Chemical Science ( IF 8.4 ) Pub Date : 2018-11-26 00:00:00 , DOI: 10.1039/c8sc04386h Tasuku Hirayama 1, 2, 3, 4 , Masatoshi Inden 2, 3, 4, 5 , Hitomi Tsuboi 1, 2, 3, 4 , Masato Niwa 1, 2, 3, 4 , Yasuhiro Uchida 2, 3, 4, 5 , Yuki Naka 2, 3, 4, 5 , Isao Hozumi 2, 3, 4, 5 , Hideko Nagasawa 1, 2, 3, 4
Chemical Science ( IF 8.4 ) Pub Date : 2018-11-26 00:00:00 , DOI: 10.1039/c8sc04386h Tasuku Hirayama 1, 2, 3, 4 , Masatoshi Inden 2, 3, 4, 5 , Hitomi Tsuboi 1, 2, 3, 4 , Masato Niwa 1, 2, 3, 4 , Yasuhiro Uchida 2, 3, 4, 5 , Yuki Naka 2, 3, 4, 5 , Isao Hozumi 2, 3, 4, 5 , Hideko Nagasawa 1, 2, 3, 4
Affiliation
|
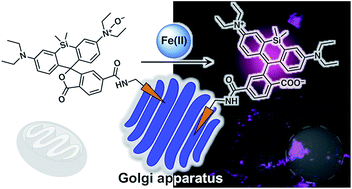
Iron is involved in numerous physiologically essential processes in our body. However, excessive iron is a pathogenic factor in neurodegenerative diseases, causing aberrant oxidative stress. Divalent metal transporter 1 (DMT1) acts as a primary transporter of Fe(II) ions. The intracellular delivery of DMT1 toward the cellular membrane via the trans-Golgi network during the endocytotic process is partially regulated by a retromer-mediated protein-sorting system comprising vacuolar protein-sorting proteins (VPSs). Thus, together with DMT1, the Golgi-apparatus acts as a hub organelle in the delivery system for intracellular Fe(II) ions. Dysfunction of the VPS-relevant protein sorting system can induce the abnormal delivery of DMT1 toward lysosomes concomitantly with Fe(II) ions. To explore this issue, we developed a fluorescent probe, Gol-SiRhoNox, for the Golgi-specific detection of Fe(II) ions by integrating our original N-oxide-based Fe(II)-specific chemical switch, a new Golgi-localizable chemical motif, and polarity-sensitive fluorogenic scaffold. Our synchronous imaging study using Gol-SiRhoNox and LysoRhoNox, a previously developed fluorescent probe for lysosomal Fe(II), revealed that the intracellular distribution balance of Fe(II) ions between the Golgi apparatus and lysosomes is normally Golgi-dominant, whereas the lysosome-specific elevation of Fe(II) ions was observed in cells with induced dysfunction of VPS35, a member of the retromer complex. Treatment of cells with dysfunctional VPS35 with R55, a molecular chaperone, resulted in the restoration of the subcellular distribution of Fe(II) ions to the Golgi-dominant state. These results indicate that the impairment of the DMT1 traffic machinery affects subcellular iron homeostasis, promoting Fe(II) leakage at the Golgi and lysosomal accumulation of Fe(II) through missorting of DMT1.
中文翻译:

高尔基体靶向的荧光探针,用于检测不稳定的Fe(ii),以揭示由VPS35功能障碍引起的异常细胞铁分布†
铁参与人体许多生理上必不可少的过程。然而,过量的铁是神经退行性疾病中的致病因素,导致异常的氧化应激。二价金属转运蛋白1(DMT1)充当Fe(II)离子的主要转运蛋白。在内吞过程中,DMT1通过反式高尔基网络向细胞膜的细胞内传递受包含液泡蛋白分选蛋白(VPS)的逆转录介导的蛋白分选系统部分调节。因此,高尔基体与DMT1一起在细胞内Fe(II)离子。VPS相关蛋白分选系统的功能异常会导致DMT1与Fe(II)离子向溶酶体的异常递送。为了探索这个问题,我们开发了一种荧光探针Gol-SiRhoNox,它通过整合我们原始的基于N-氧化物的Fe(II)专用化学开关(一种新的Golgi本地化),用于高尔基体特定的Fe(II)离子检测。化学基序和对极性敏感的荧光支架。使用高尔-SiRhoNox和LysoRhoNox,溶酶体的Fe先前开发的荧光探针(我们的同步成像研究II),表明Fe的细胞内分布平衡(II)离子在高尔基体和溶酶体之间通常以高尔基体为主,而在具有诱导型VPS35功能障碍(逆转录复合物的成员)的细胞中观察到了Fe(II)离子的溶酶体特异性升高。用功能性分子伴侣R55对功能失调的VPS35处理的细胞可将Fe(II)离子的亚细胞分布恢复到高尔基占优势状态。这些结果表明DMT1交通机械的损害会影响亚细胞铁稳态,通过错配DMT1促进高尔基体Fe(II)泄漏和溶酶体积累Fe(II)。
更新日期:2018-11-26
中文翻译:

高尔基体靶向的荧光探针,用于检测不稳定的Fe(ii),以揭示由VPS35功能障碍引起的异常细胞铁分布†
铁参与人体许多生理上必不可少的过程。然而,过量的铁是神经退行性疾病中的致病因素,导致异常的氧化应激。二价金属转运蛋白1(DMT1)充当Fe(II)离子的主要转运蛋白。在内吞过程中,DMT1通过反式高尔基网络向细胞膜的细胞内传递受包含液泡蛋白分选蛋白(VPS)的逆转录介导的蛋白分选系统部分调节。因此,高尔基体与DMT1一起在细胞内Fe(II)离子。VPS相关蛋白分选系统的功能异常会导致DMT1与Fe(II)离子向溶酶体的异常递送。为了探索这个问题,我们开发了一种荧光探针Gol-SiRhoNox,它通过整合我们原始的基于N-氧化物的Fe(II)专用化学开关(一种新的Golgi本地化),用于高尔基体特定的Fe(II)离子检测。化学基序和对极性敏感的荧光支架。使用高尔-SiRhoNox和LysoRhoNox,溶酶体的Fe先前开发的荧光探针(我们的同步成像研究II),表明Fe的细胞内分布平衡(II)离子在高尔基体和溶酶体之间通常以高尔基体为主,而在具有诱导型VPS35功能障碍(逆转录复合物的成员)的细胞中观察到了Fe(II)离子的溶酶体特异性升高。用功能性分子伴侣R55对功能失调的VPS35处理的细胞可将Fe(II)离子的亚细胞分布恢复到高尔基占优势状态。这些结果表明DMT1交通机械的损害会影响亚细胞铁稳态,通过错配DMT1促进高尔基体Fe(II)泄漏和溶酶体积累Fe(II)。


























 京公网安备 11010802027423号
京公网安备 11010802027423号