当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
An ONIOM investigation of the effect of conformation on bond dissociation energies in peptides
Journal of Computational Chemistry ( IF 3 ) Pub Date : 2018-11-19 , DOI: 10.1002/jcc.25538 Bun Chan 1 , Leo Radom 2
Journal of Computational Chemistry ( IF 3 ) Pub Date : 2018-11-19 , DOI: 10.1002/jcc.25538 Bun Chan 1 , Leo Radom 2
Affiliation
|
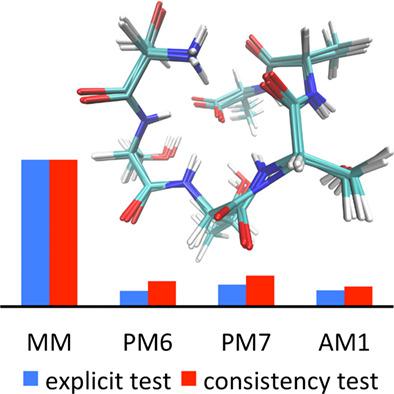
In the present study, we use the ONIOM strategy of Morokuma and coworkers to examine the various CH bond dissociation energies (BDEs) of a small peptide (2ONW) and compare these with values obtained for its component individual amino acid residues. To evaluate suitable methods for ONIOM‐based geometry optimizations, we test an “internal consistency” approach against full B3‐LYP//B3‐LYP results, and find B3‐LYP/6‐31G(d):AM1 to be appropriate. We find that the BDEs at the α‐carbon in 2ONW are generally larger than the corresponding values for the individual residues on their own. This is attributed to the constraints of the peptide backbone leading to conformations that are not ideal for captodative stabilization of the resulting α‐radicals. At the more flexible β‐ and γ‐positions, the differences between the BDEs in 2ONW and the individual residues are smaller. Overall, the α‐BDEs are smaller than the β‐ and γ‐BDEs in most cases. Thus, to rationalize the inertness of peptide backbones with respect to α‐hydrogen abstraction that is frequently found experimentally, it is necessary to consider alternative protection mechanisms such as the polar effect. © 2018 Wiley Periodicals, Inc.
中文翻译:

构象对肽键解离能影响的 ONIOM 研究
在本研究中,我们使用 Morokuma 及其同事的 ONIOM 策略来检查小肽 (2ONW) 的各种 CH 键解离能 (BDE),并将这些值与其组分单个氨基酸残基的值进行比较。为了评估基于 ONIOM 的几何优化的合适方法,我们针对完整的 B3-LYP//B3-LYP 结果测试了“内部一致性”方法,并发现 B3-LYP/6-31G(d):AM1 是合适的。我们发现 2ONW 中 α-碳上的 BDE 通常大于单个残基本身的相应值。这是由于肽主链的限制导致构象对于所得α-自由基的俘获稳定化不理想。在更灵活的 β 和 γ 位置,2ONW 中的 BDEs 和单个残留物之间的差异较小。总的来说,在大多数情况下,α-BDEs 比 β-BDEs 和 γ-BDEs 小。因此,为了在实验中经常发现的 α-氢提取方面使肽骨架的惰性合理化,有必要考虑替代保护机制,例如极性效应。© 2018 Wiley Periodicals, Inc.
更新日期:2018-11-19
中文翻译:

构象对肽键解离能影响的 ONIOM 研究
在本研究中,我们使用 Morokuma 及其同事的 ONIOM 策略来检查小肽 (2ONW) 的各种 CH 键解离能 (BDE),并将这些值与其组分单个氨基酸残基的值进行比较。为了评估基于 ONIOM 的几何优化的合适方法,我们针对完整的 B3-LYP//B3-LYP 结果测试了“内部一致性”方法,并发现 B3-LYP/6-31G(d):AM1 是合适的。我们发现 2ONW 中 α-碳上的 BDE 通常大于单个残基本身的相应值。这是由于肽主链的限制导致构象对于所得α-自由基的俘获稳定化不理想。在更灵活的 β 和 γ 位置,2ONW 中的 BDEs 和单个残留物之间的差异较小。总的来说,在大多数情况下,α-BDEs 比 β-BDEs 和 γ-BDEs 小。因此,为了在实验中经常发现的 α-氢提取方面使肽骨架的惰性合理化,有必要考虑替代保护机制,例如极性效应。© 2018 Wiley Periodicals, Inc.



























 京公网安备 11010802027423号
京公网安备 11010802027423号