当前位置:
X-MOL 学术
›
Acta Pharmacol. Sin.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
THZ1 suppresses human non-small-cell lung cancer cells in vitro through interference with cancer metabolism.
Acta Pharmacologica Sinica ( IF 8.2 ) Pub Date : 2018-11-16 , DOI: 10.1038/s41401-018-0187-3 Zhu-Jun Cheng 1 , Du-Ling Miao 1 , Qiu-Yun Su 2 , Xiao-Li Tang 2 , Xiao-Lei Wang 1 , Li-Bin Deng 1 , Hui-Dong Shi 1, 3 , Hong-Bo Xin 1
Acta Pharmacologica Sinica ( IF 8.2 ) Pub Date : 2018-11-16 , DOI: 10.1038/s41401-018-0187-3 Zhu-Jun Cheng 1 , Du-Ling Miao 1 , Qiu-Yun Su 2 , Xiao-Li Tang 2 , Xiao-Lei Wang 1 , Li-Bin Deng 1 , Hui-Dong Shi 1, 3 , Hong-Bo Xin 1
Affiliation
|
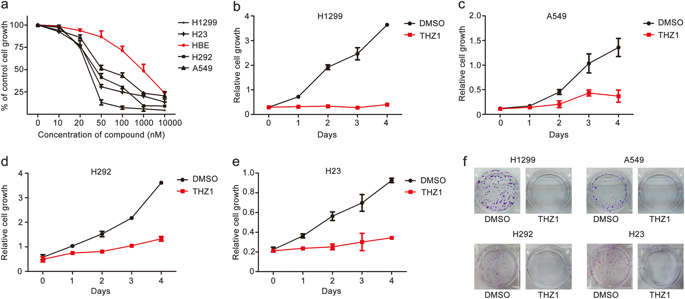
Cancer cells always require more nutrients, energy, and biosynthetic activity to sustain their rapid proliferation than normal cells. Previous studies have shown the impact of THZ1, a covalent inhibitor of cyclin-dependent kinase 7 (CDK7), on transcription regulation and cell-cycle arrest in numerous cancers, but its effects on cellular metabolism in cancer cells remain unknown. In this study we elucidated the anticancer mechanism of THZ1 in human non-small-cell lung cancer (NSCLC) cells. We showed that treatment with THZ1 (10-1000 nM) dose-dependently suppressed the proliferation of human NSCLC cell lines H1299, A549, H292, and H23, and markedly inhibited the migration of these NSCLC cells. Furthermore, treatment with THZ1 (50 nM) arrested cell cycle at G2/M phase and induced apoptosis in these NSCLC cell lines. More importantly, we revealed that treatment with THZ1 (50 nM) blocked the glycolysis pathway but had no effect on glutamine metabolism. We further demonstrated that THZ1 treatment altered the expression pattern of glutaminase 1 (GLS1) isoforms through promoting the ubiquitination and degradation of NUDT21. Combined treatment of THZ1 with a glutaminase inhibitor CB-839 (500 nM) exerted a more potent anti-proliferative effect in these NSCLC cell lines than treatment with THZ1 or CB-839 alone. Our results demonstrate that the inhibitory effect of THZ1 on the growth of human NSCLC cells is partially attributed to interfering with cancer metabolism. Thus, we provide a new potential therapeutic strategy for NSCLC treatment by combining THZ1 with the inhibitors of glutamine metabolism.
中文翻译:

THZ1通过干扰癌症新陈代谢,在体外抑制人非小细胞肺癌细胞。
癌细胞总是比正常细胞需要更多的营养,能量和生物合成活性来维持其快速增殖。先前的研究表明,THZ1是细胞周期蛋白依赖性激酶7(CDK7)的一种共价抑制剂,对许多癌症的转录调控和细胞周期阻滞具有影响,但其对癌细胞中细胞代谢的影响仍然未知。在这项研究中,我们阐明了THZ1在人非小细胞肺癌(NSCLC)细胞中的抗癌机制。我们显示,用THZ1(10-1000 nM)进行剂量依赖性地抑制人NSCLC细胞系H1299,A549,H292和H23的增殖,并显着抑制了这些NSCLC细胞的迁移。此外,用THZ1(50 nM)进行的治疗使细胞周期停滞在G2 / M期,并诱导了这些NSCLC细胞系的凋亡。更重要的是,我们发现用THZ1(50 nM)的治疗可阻断糖酵解途径,但对谷氨酰胺代谢没有影响。我们进一步证明了THZ1处理可通过促进NUDT21的泛素化和降解来改变谷氨酰胺酶1(GLS1)亚型的表达模式。与单独使用THZ1或CB-839的治疗相比,将THZ1与谷氨酰胺酶抑制剂CB-839(500 nM)的联合治疗在这些NSCLC细胞系中发挥了更有效的抗增殖作用。我们的结果表明,THZ1对人NSCLC细胞生长的抑制作用部分归因于干扰癌症代谢。因此,我们通过将THZ1与谷氨酰胺代谢抑制剂相结合,为NSCLC治疗提供了一种新的潜在治疗策略。
更新日期:2019-01-26
中文翻译:

THZ1通过干扰癌症新陈代谢,在体外抑制人非小细胞肺癌细胞。
癌细胞总是比正常细胞需要更多的营养,能量和生物合成活性来维持其快速增殖。先前的研究表明,THZ1是细胞周期蛋白依赖性激酶7(CDK7)的一种共价抑制剂,对许多癌症的转录调控和细胞周期阻滞具有影响,但其对癌细胞中细胞代谢的影响仍然未知。在这项研究中,我们阐明了THZ1在人非小细胞肺癌(NSCLC)细胞中的抗癌机制。我们显示,用THZ1(10-1000 nM)进行剂量依赖性地抑制人NSCLC细胞系H1299,A549,H292和H23的增殖,并显着抑制了这些NSCLC细胞的迁移。此外,用THZ1(50 nM)进行的治疗使细胞周期停滞在G2 / M期,并诱导了这些NSCLC细胞系的凋亡。更重要的是,我们发现用THZ1(50 nM)的治疗可阻断糖酵解途径,但对谷氨酰胺代谢没有影响。我们进一步证明了THZ1处理可通过促进NUDT21的泛素化和降解来改变谷氨酰胺酶1(GLS1)亚型的表达模式。与单独使用THZ1或CB-839的治疗相比,将THZ1与谷氨酰胺酶抑制剂CB-839(500 nM)的联合治疗在这些NSCLC细胞系中发挥了更有效的抗增殖作用。我们的结果表明,THZ1对人NSCLC细胞生长的抑制作用部分归因于干扰癌症代谢。因此,我们通过将THZ1与谷氨酰胺代谢抑制剂相结合,为NSCLC治疗提供了一种新的潜在治疗策略。


























 京公网安备 11010802027423号
京公网安备 11010802027423号