当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Catalytic activity of new heteroleptic [Cu(PPh3)2(β-oxodithioester)] complexes: click derived triazolyl glycoconjugates†‡
New Journal of Chemistry ( IF 3.3 ) Pub Date : 2018-11-15 00:00:00 , DOI: 10.1039/c8nj05075a Kavita Kumari 1, 2, 3, 4, 5 , Anoop S. Singh 1, 2, 3, 4, 5 , Krishna K. Manar 1, 2, 3, 4, 5 , Chote Lal Yadav 1, 2, 3, 4, 5 , Vinod K. Tiwari 1, 2, 3, 4, 5 , Michael G. B. Drew 1, 6, 7, 8, 9 , Nanhai Singh 1, 2, 3, 4, 5
New Journal of Chemistry ( IF 3.3 ) Pub Date : 2018-11-15 00:00:00 , DOI: 10.1039/c8nj05075a Kavita Kumari 1, 2, 3, 4, 5 , Anoop S. Singh 1, 2, 3, 4, 5 , Krishna K. Manar 1, 2, 3, 4, 5 , Chote Lal Yadav 1, 2, 3, 4, 5 , Vinod K. Tiwari 1, 2, 3, 4, 5 , Michael G. B. Drew 1, 6, 7, 8, 9 , Nanhai Singh 1, 2, 3, 4, 5
Affiliation
|
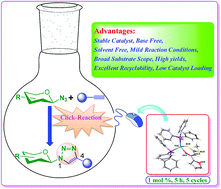
A series of four new and two known luminescent heteroleptic Cu(I) complexes of the form [Cu(PPh3)2(β-oxodithioester)] (β-oxodithioester = methyl-3-hydroxy-3-(2-furyl)-2-propenedithioate L1 1, methyl-3-hydroxy-3-(2-thienyl)-2-propenedithioate L2 2, methyl-3-hydroxy-3-(4-methoxyphenyl)-2-propenedithioate L3 3, methyl-3-hydroxy-3-(4-bromophenyl)-2-propenedithioate L4 4, methyl-3-hydroxy-3-benzyl-2-propenedithioate L5 5 and methyl-3-hydroxy-3-(3-pyridyl)-2-propenedithioate, L6 6) have been synthesized and characterized by elemental (C, H, N) analysis, and IR, UV-visible, 1H, 13C{1H}, and 31P{1H} NMR spectroscopy and their structures have been ascertained by X-ray crystallography. These complexes were exploited as catalysts for azide–alkyne cycloaddition reactions (click chemistry) for the synthesis of triazolyl glycoconjugates, where they displayed efficient catalytic activity at room temperature. Optimization of the reaction conditions and formation of regioselective products in high yields in the absence of a base/additive are concomitant with the click protocol. The substrate scope was successfully extended by varying the types of sugar azides and alkyne scaffolds, affording the corresponding triazole products in excellent yields at low catalyst loadings (1 mol%). Furthermore, catalyst 1 showed excellent recyclability and was reused for five cycles with little decrease in efficiency.
中文翻译:

新型杂合[Cu(PPh 3)2(β-氧二硫代酯)]配合物的催化活性:单击衍生的三唑基糖缀合物† ‡
[Cu(PPh 3)2(β-oxodithioester)]形式的一系列四个新的和两个已知的发光杂铜Cu(I)配合物(β-oxodithioester=甲基-3-羟基-3-(2-呋喃基)- 2-丙二硫代酸酯L1 1,-3-羟基-3-(2-噻吩基)-2-丙二硫代酸甲酯L2 2,-3-羟基-3-(4-甲氧基苯基)-2-丙二硫代酸甲酯L3 3,甲基-3-羟基-3-(4-溴苯基)-2-丙二硫代酸酯L4 4,甲基-3-羟基-3-苄基-2-丙二硫代酸酯L5 5和-3-羟基-3-(3-吡啶基)-2-丙二硫代甲基酯, L6 6)已合成,并通过元素(C,H,N)分析和IR,UV可见,1 H,通过X射线晶体学确定了13 C { 1 H}和31 P { 1 H} NMR光谱及其结构。这些络合物被用作叠氮基-炔烃环加成反应(点击化学)的催化剂,用于合成三唑基糖缀合物,在室温下它们表现出有效的催化活性。在没有碱/添加剂的情况下,优化反应条件并以高收率形成区域选择性产物与点击方案同时进行。通过改变糖叠氮化物和炔烃骨架的类型,成功扩大了底物范围,从而在低催化剂负载量(1摩尔%)下以优异的收率提供了相应的三唑产品。此外,催化剂1 具有良好的可回收性,可重复使用五个周期,效率几乎没有下降。
更新日期:2018-11-15
中文翻译:

新型杂合[Cu(PPh 3)2(β-氧二硫代酯)]配合物的催化活性:单击衍生的三唑基糖缀合物† ‡
[Cu(PPh 3)2(β-oxodithioester)]形式的一系列四个新的和两个已知的发光杂铜Cu(I)配合物(β-oxodithioester=甲基-3-羟基-3-(2-呋喃基)- 2-丙二硫代酸酯L1 1,-3-羟基-3-(2-噻吩基)-2-丙二硫代酸甲酯L2 2,-3-羟基-3-(4-甲氧基苯基)-2-丙二硫代酸甲酯L3 3,甲基-3-羟基-3-(4-溴苯基)-2-丙二硫代酸酯L4 4,甲基-3-羟基-3-苄基-2-丙二硫代酸酯L5 5和-3-羟基-3-(3-吡啶基)-2-丙二硫代甲基酯, L6 6)已合成,并通过元素(C,H,N)分析和IR,UV可见,1 H,通过X射线晶体学确定了13 C { 1 H}和31 P { 1 H} NMR光谱及其结构。这些络合物被用作叠氮基-炔烃环加成反应(点击化学)的催化剂,用于合成三唑基糖缀合物,在室温下它们表现出有效的催化活性。在没有碱/添加剂的情况下,优化反应条件并以高收率形成区域选择性产物与点击方案同时进行。通过改变糖叠氮化物和炔烃骨架的类型,成功扩大了底物范围,从而在低催化剂负载量(1摩尔%)下以优异的收率提供了相应的三唑产品。此外,催化剂1 具有良好的可回收性,可重复使用五个周期,效率几乎没有下降。


























 京公网安备 11010802027423号
京公网安备 11010802027423号