当前位置:
X-MOL 学术
›
Chem. Asian J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Recent Advances in the Metal‐Catalyzed Activation of Amide Bonds
Chemistry - An Asian Journal ( IF 4.1 ) Pub Date : 2018-12-06 , DOI: 10.1002/asia.201801317 Moreshwar B. Chaudhari 1 , Boopathy Gnanaprakasam 1
Chemistry - An Asian Journal ( IF 4.1 ) Pub Date : 2018-12-06 , DOI: 10.1002/asia.201801317 Moreshwar B. Chaudhari 1 , Boopathy Gnanaprakasam 1
Affiliation
|
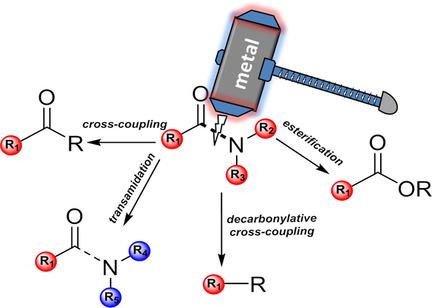
The amide functional group is commonly found in peptides, proteins, pharmaceutical compounds, natural products, and polymers. The synthesis of amides is typically performed by using classical approaches that involve the reaction between a carboxylic acid and an amine in the presence of an activator. Amides are thought to be an inert functional group, because they are unsusceptible to nucleophile attack, owing to their low electrophilicity. The reason for this resistance is clear: the resonance stability of the amide bond. However, transition metal catalysis can circumvent this stability by selectively rupturing the N−C bond of the amide, thereby facilitating further cross‐coupling or other reactions. In this Focus Review, we discuss the recent advances in this area and present a summary of methods that have been developed for activating the amide N−C bond by using precious and non‐precious metals.
中文翻译:

金属催化酰胺键活化的最新进展
酰胺官能团通常存在于肽,蛋白质,药物化合物,天然产物和聚合物中。酰胺的合成通常通过使用经典方法进行,该经典方法涉及在活化剂存在下羧酸与胺之间的反应。酰胺被认为是惰性的官能团,因为它们由于亲电性低而对亲核试剂不敏感。产生这种抗性的原因很明显:酰胺键的共振稳定性。但是,过渡金属催化可以通过选择性地破坏酰胺的N-C键来破坏这种稳定性,从而促进进一步的交叉偶联或其他反应。在本焦点回顾中,
更新日期:2018-12-06
中文翻译:

金属催化酰胺键活化的最新进展
酰胺官能团通常存在于肽,蛋白质,药物化合物,天然产物和聚合物中。酰胺的合成通常通过使用经典方法进行,该经典方法涉及在活化剂存在下羧酸与胺之间的反应。酰胺被认为是惰性的官能团,因为它们由于亲电性低而对亲核试剂不敏感。产生这种抗性的原因很明显:酰胺键的共振稳定性。但是,过渡金属催化可以通过选择性地破坏酰胺的N-C键来破坏这种稳定性,从而促进进一步的交叉偶联或其他反应。在本焦点回顾中,



























 京公网安备 11010802027423号
京公网安备 11010802027423号