Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Autophagy maintains tumour growth through circulating arginine
Nature ( IF 64.8 ) Pub Date : 2018-11-01 , DOI: 10.1038/s41586-018-0697-7 Laura Poillet-Perez , Xiaoqi Xie , Le Zhan , Yang Yang , Daniel W. Sharp , Zhixian Sherrie Hu , Xiaoyang Su , Anurag Maganti , Cherry Jiang , Wenyun Lu , Haiyan Zheng , Marcus W. Bosenberg , Janice M. Mehnert , Jessie Yanxiang Guo , Edmund Lattime , Joshua D. Rabinowitz , Eileen White
Nature ( IF 64.8 ) Pub Date : 2018-11-01 , DOI: 10.1038/s41586-018-0697-7 Laura Poillet-Perez , Xiaoqi Xie , Le Zhan , Yang Yang , Daniel W. Sharp , Zhixian Sherrie Hu , Xiaoyang Su , Anurag Maganti , Cherry Jiang , Wenyun Lu , Haiyan Zheng , Marcus W. Bosenberg , Janice M. Mehnert , Jessie Yanxiang Guo , Edmund Lattime , Joshua D. Rabinowitz , Eileen White
|
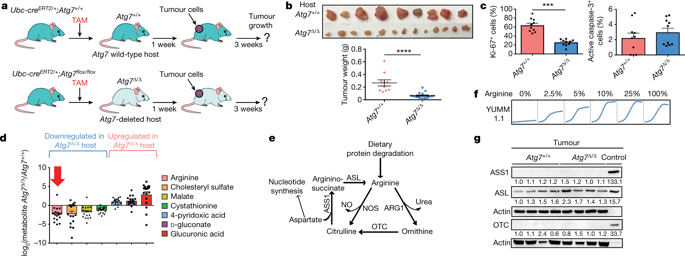
Autophagy captures intracellular components and delivers them to lysosomes, where they are degraded and recycled to sustain metabolism and to enable survival during starvation1–5. Acute, whole-body deletion of the essential autophagy gene Atg7 in adult mice causes a systemic metabolic defect that manifests as starvation intolerance and gradual loss of white adipose tissue, liver glycogen and muscle mass1. Cancer cells also benefit from autophagy. Deletion of essential autophagy genes impairs the metabolism, proliferation, survival and malignancy of spontaneous tumours in models of autochthonous cancer6,7. Acute, systemic deletion of Atg7 or acute, systemic expression of a dominant-negative ATG4b in mice induces greater regression of KRAS-driven cancers than does tumour-specific autophagy deletion, which suggests that host autophagy promotes tumour growth1,8. Here we show that host-specific deletion of Atg7 impairs the growth of multiple allografted tumours, although not all tumour lines were sensitive to host autophagy status. Loss of autophagy in the host was associated with a reduction in circulating arginine, and the sensitive tumour cell lines were arginine auxotrophs owing to the lack of expression of the enzyme argininosuccinate synthase 1. Serum proteomic analysis identified the arginine-degrading enzyme arginase I (ARG1) in the circulation of Atg7-deficient hosts, and in vivo arginine metabolic tracing demonstrated that serum arginine was degraded to ornithine. ARG1 is predominantly expressed in the liver and can be released from hepatocytes into the circulation. Liver-specific deletion of Atg7 produced circulating ARG1, and reduced both serum arginine and tumour growth. Deletion of Atg5 in the host similarly regulated circulating arginine and suppressed tumorigenesis, which demonstrates that this phenotype is specific to autophagy function rather than to deletion of Atg7. Dietary supplementation of Atg7-deficient hosts with arginine partially restored levels of circulating arginine and tumour growth. Thus, defective autophagy in the host leads to the release of ARG1 from the liver and the degradation of circulating arginine, which is essential for tumour growth; this identifies a metabolic vulnerability of cancer.Mice with whole-body or liver-specific deletion of Atg7 release circulating arginase I and have reduced levels of serum arginine, which impairs the growth of allografted arginine-auxotrophic tumours.
中文翻译:

自噬通过循环精氨酸维持肿瘤生长
自噬捕获细胞内成分并将它们输送到溶酶体,在溶酶体中它们被降解和回收以维持新陈代谢并在饥饿期间存活1-5。成年小鼠必需自噬基因 Atg7 的急性全身缺失导致全身代谢缺陷,表现为饥饿不耐受和白色脂肪组织、肝糖原和肌肉质量的逐渐丧失1。癌细胞也受益于自噬。在本土癌症模型中,必需自噬基因的缺失会损害自发性肿瘤的代谢、增殖、存活和恶性程度 6,7。与肿瘤特异性自噬缺失相比,小鼠中 Atg7 的急性、全身性缺失或显性负性 ATG4b 的急性、全身性表达可诱导 KRAS 驱动的癌症更大程度的消退,这表明宿主自噬促进肿瘤生长1,8。在这里,我们表明 Atg7 的宿主特异性缺失会损害多个同种异体移植肿瘤的生长,尽管并非所有肿瘤系都对宿主自噬状态敏感。宿主中自噬的丧失与循环精氨酸的减少有关,敏感的肿瘤细胞系由于缺乏精氨酸琥珀酸合酶 1 的表达而成为精氨酸营养缺陷型。血清蛋白质组学分析确定了精氨酸降解酶精氨酸酶 I(ARG1 ) 在 Atg7 缺陷宿主的循环中,体内精氨酸代谢追踪表明血清精氨酸被降解为鸟氨酸。ARG1 主要在肝脏中表达,可以从肝细胞释放到循环中。Atg7 的肝脏特异性缺失产生循环 ARG1,并减少血清精氨酸和肿瘤生长。宿主中 Atg5 的缺失类似地调节循环精氨酸并抑制肿瘤发生,这表明这种表型对自噬功能具有特异性,而不是对 Atg7 的缺失。用精氨酸膳食补充 Atg7 缺陷宿主可部分恢复循环精氨酸水平和肿瘤生长。因此,宿主中的自噬缺陷导致 ARG1 从肝脏中释放和循环精氨酸的降解,这是肿瘤生长所必需的;这确定了癌症的代谢脆弱性。具有全身或肝脏特异性 Atg7 缺失的小鼠会释放循环精氨酸酶 I,并且血清精氨酸水平降低,这会损害同种异体移植的精氨酸营养缺陷型肿瘤的生长。宿主中 Atg5 的缺失类似地调节循环精氨酸并抑制肿瘤发生,这表明这种表型对自噬功能具有特异性,而不是对 Atg7 的缺失。用精氨酸膳食补充 Atg7 缺陷宿主可部分恢复循环精氨酸水平和肿瘤生长。因此,宿主中存在缺陷的自噬导致 ARG1 从肝脏中释放和循环精氨酸的降解,这是肿瘤生长所必需的;这确定了癌症的代谢脆弱性。具有全身或肝脏特异性 Atg7 缺失的小鼠会释放循环精氨酸酶 I,并且血清精氨酸水平降低,这会损害同种异体移植的精氨酸营养缺陷型肿瘤的生长。宿主中 Atg5 的缺失类似地调节循环精氨酸并抑制肿瘤发生,这表明这种表型对自噬功能具有特异性,而不是对 Atg7 的缺失。用精氨酸膳食补充 Atg7 缺陷宿主可部分恢复循环精氨酸水平和肿瘤生长。因此,宿主中存在缺陷的自噬导致 ARG1 从肝脏中释放和循环精氨酸的降解,这是肿瘤生长所必需的;这确定了癌症的代谢脆弱性。具有全身或肝脏特异性 Atg7 缺失的小鼠会释放循环精氨酸酶 I,并且血清精氨酸水平降低,这会损害同种异体移植的精氨酸营养缺陷型肿瘤的生长。这表明这种表型是自噬功能特异性的,而不是 Atg7 的缺失。用精氨酸膳食补充 Atg7 缺陷宿主可部分恢复循环精氨酸水平和肿瘤生长。因此,宿主中存在缺陷的自噬导致 ARG1 从肝脏中释放和循环精氨酸的降解,这是肿瘤生长所必需的;这确定了癌症的代谢脆弱性。具有全身或肝脏特异性 Atg7 缺失的小鼠会释放循环精氨酸酶 I,并且血清精氨酸水平降低,这会损害同种异体移植的精氨酸营养缺陷型肿瘤的生长。这表明这种表型是自噬功能特异性的,而不是 Atg7 的缺失。用精氨酸膳食补充 Atg7 缺陷宿主可部分恢复循环精氨酸水平和肿瘤生长。因此,宿主中存在缺陷的自噬导致 ARG1 从肝脏中释放和循环精氨酸的降解,这是肿瘤生长所必需的;这确定了癌症的代谢脆弱性。具有全身或肝脏特异性 Atg7 缺失的小鼠会释放循环精氨酸酶 I,并且血清精氨酸水平降低,这会损害同种异体移植的精氨酸营养缺陷型肿瘤的生长。用精氨酸膳食补充 Atg7 缺陷宿主可部分恢复循环精氨酸水平和肿瘤生长。因此,宿主中的自噬缺陷导致 ARG1 从肝脏中释放和循环精氨酸的降解,这是肿瘤生长所必需的;这确定了癌症的代谢脆弱性。具有全身或肝脏特异性 Atg7 缺失的小鼠会释放循环精氨酸酶 I,并且血清精氨酸水平降低,这会损害同种异体移植的精氨酸营养缺陷型肿瘤的生长。用精氨酸膳食补充 Atg7 缺陷宿主可部分恢复循环精氨酸水平和肿瘤生长。因此,宿主中存在缺陷的自噬导致 ARG1 从肝脏中释放和循环精氨酸的降解,这是肿瘤生长所必需的;这确定了癌症的代谢脆弱性。具有全身或肝脏特异性 Atg7 缺失的小鼠会释放循环精氨酸酶 I,并且血清精氨酸水平降低,这会损害同种异体移植的精氨酸营养缺陷型肿瘤的生长。
更新日期:2018-11-01
中文翻译:

自噬通过循环精氨酸维持肿瘤生长
自噬捕获细胞内成分并将它们输送到溶酶体,在溶酶体中它们被降解和回收以维持新陈代谢并在饥饿期间存活1-5。成年小鼠必需自噬基因 Atg7 的急性全身缺失导致全身代谢缺陷,表现为饥饿不耐受和白色脂肪组织、肝糖原和肌肉质量的逐渐丧失1。癌细胞也受益于自噬。在本土癌症模型中,必需自噬基因的缺失会损害自发性肿瘤的代谢、增殖、存活和恶性程度 6,7。与肿瘤特异性自噬缺失相比,小鼠中 Atg7 的急性、全身性缺失或显性负性 ATG4b 的急性、全身性表达可诱导 KRAS 驱动的癌症更大程度的消退,这表明宿主自噬促进肿瘤生长1,8。在这里,我们表明 Atg7 的宿主特异性缺失会损害多个同种异体移植肿瘤的生长,尽管并非所有肿瘤系都对宿主自噬状态敏感。宿主中自噬的丧失与循环精氨酸的减少有关,敏感的肿瘤细胞系由于缺乏精氨酸琥珀酸合酶 1 的表达而成为精氨酸营养缺陷型。血清蛋白质组学分析确定了精氨酸降解酶精氨酸酶 I(ARG1 ) 在 Atg7 缺陷宿主的循环中,体内精氨酸代谢追踪表明血清精氨酸被降解为鸟氨酸。ARG1 主要在肝脏中表达,可以从肝细胞释放到循环中。Atg7 的肝脏特异性缺失产生循环 ARG1,并减少血清精氨酸和肿瘤生长。宿主中 Atg5 的缺失类似地调节循环精氨酸并抑制肿瘤发生,这表明这种表型对自噬功能具有特异性,而不是对 Atg7 的缺失。用精氨酸膳食补充 Atg7 缺陷宿主可部分恢复循环精氨酸水平和肿瘤生长。因此,宿主中的自噬缺陷导致 ARG1 从肝脏中释放和循环精氨酸的降解,这是肿瘤生长所必需的;这确定了癌症的代谢脆弱性。具有全身或肝脏特异性 Atg7 缺失的小鼠会释放循环精氨酸酶 I,并且血清精氨酸水平降低,这会损害同种异体移植的精氨酸营养缺陷型肿瘤的生长。宿主中 Atg5 的缺失类似地调节循环精氨酸并抑制肿瘤发生,这表明这种表型对自噬功能具有特异性,而不是对 Atg7 的缺失。用精氨酸膳食补充 Atg7 缺陷宿主可部分恢复循环精氨酸水平和肿瘤生长。因此,宿主中存在缺陷的自噬导致 ARG1 从肝脏中释放和循环精氨酸的降解,这是肿瘤生长所必需的;这确定了癌症的代谢脆弱性。具有全身或肝脏特异性 Atg7 缺失的小鼠会释放循环精氨酸酶 I,并且血清精氨酸水平降低,这会损害同种异体移植的精氨酸营养缺陷型肿瘤的生长。宿主中 Atg5 的缺失类似地调节循环精氨酸并抑制肿瘤发生,这表明这种表型对自噬功能具有特异性,而不是对 Atg7 的缺失。用精氨酸膳食补充 Atg7 缺陷宿主可部分恢复循环精氨酸水平和肿瘤生长。因此,宿主中存在缺陷的自噬导致 ARG1 从肝脏中释放和循环精氨酸的降解,这是肿瘤生长所必需的;这确定了癌症的代谢脆弱性。具有全身或肝脏特异性 Atg7 缺失的小鼠会释放循环精氨酸酶 I,并且血清精氨酸水平降低,这会损害同种异体移植的精氨酸营养缺陷型肿瘤的生长。这表明这种表型是自噬功能特异性的,而不是 Atg7 的缺失。用精氨酸膳食补充 Atg7 缺陷宿主可部分恢复循环精氨酸水平和肿瘤生长。因此,宿主中存在缺陷的自噬导致 ARG1 从肝脏中释放和循环精氨酸的降解,这是肿瘤生长所必需的;这确定了癌症的代谢脆弱性。具有全身或肝脏特异性 Atg7 缺失的小鼠会释放循环精氨酸酶 I,并且血清精氨酸水平降低,这会损害同种异体移植的精氨酸营养缺陷型肿瘤的生长。这表明这种表型是自噬功能特异性的,而不是 Atg7 的缺失。用精氨酸膳食补充 Atg7 缺陷宿主可部分恢复循环精氨酸水平和肿瘤生长。因此,宿主中存在缺陷的自噬导致 ARG1 从肝脏中释放和循环精氨酸的降解,这是肿瘤生长所必需的;这确定了癌症的代谢脆弱性。具有全身或肝脏特异性 Atg7 缺失的小鼠会释放循环精氨酸酶 I,并且血清精氨酸水平降低,这会损害同种异体移植的精氨酸营养缺陷型肿瘤的生长。用精氨酸膳食补充 Atg7 缺陷宿主可部分恢复循环精氨酸水平和肿瘤生长。因此,宿主中的自噬缺陷导致 ARG1 从肝脏中释放和循环精氨酸的降解,这是肿瘤生长所必需的;这确定了癌症的代谢脆弱性。具有全身或肝脏特异性 Atg7 缺失的小鼠会释放循环精氨酸酶 I,并且血清精氨酸水平降低,这会损害同种异体移植的精氨酸营养缺陷型肿瘤的生长。用精氨酸膳食补充 Atg7 缺陷宿主可部分恢复循环精氨酸水平和肿瘤生长。因此,宿主中存在缺陷的自噬导致 ARG1 从肝脏中释放和循环精氨酸的降解,这是肿瘤生长所必需的;这确定了癌症的代谢脆弱性。具有全身或肝脏特异性 Atg7 缺失的小鼠会释放循环精氨酸酶 I,并且血清精氨酸水平降低,这会损害同种异体移植的精氨酸营养缺陷型肿瘤的生长。



























 京公网安备 11010802027423号
京公网安备 11010802027423号