当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Biobased Chemicals: 1,2,4-Benzenetriol, Selective Deuteration and Dimerization to Bifunctional Aromatic Compounds
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2018-11-09 00:00:00 , DOI: 10.1021/acs.oprd.8b00303 Caelan Randolph 1 , Ciaran W. Lahive 1 , Selim Sami 2, 3 , Remco W. A. Havenith 2, 3, 4 , Hero J. Heeres 1 , Peter J. Deuss 1
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2018-11-09 00:00:00 , DOI: 10.1021/acs.oprd.8b00303 Caelan Randolph 1 , Ciaran W. Lahive 1 , Selim Sami 2, 3 , Remco W. A. Havenith 2, 3, 4 , Hero J. Heeres 1 , Peter J. Deuss 1
Affiliation
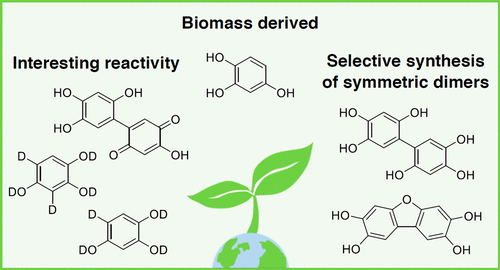
|
1,2,4-Benzenetriol (BTO), sourced from the carbohydrate-derived platform chemical 5-hydroxylmethylfurfural (HMF), is an interesting starting point for the synthesis of various biobased aromatic products. However, BTO readily undergoes dimerization and other reactions under mild conditions, making analysis and isolation challenging. To both control and utilize the reactivity of BTO to produce biobased building blocks, its reactivity needs to be better understood. Here it was found that specific BTO aromatic C–H bonds are reactive toward deuterium exchange with D2O, which appears pronounced under acidic conditions at room temperature and can lead to the selective formation of BTO with an aromatic ring that contains one or two deuterium atoms, the first at the five and the second at the three position. By exposure to air, it was shown that BTO forms a 5,5′-linked BTO dimer [1,1′-biphenyl]-2,2′,4,4′,5,5′-hexaol (1) and subsequently a hydroxyquinone containing dimeric structure 2′,4,4′,5′-tetrahydroxy-[1,1′-biphenyl]-2,5-dione (2). Additionally, condensed dimer dibenzo[b,d]furan-2,3,7,8-tetraol (3) can be relatively easily accessed. The controlled formation of these symmetric and asymmetric multifunctional dimers illustrates diverse possibilities for BTO to be converted to valuable biobased aromatic compounds. Deuterium exchange was attributed to electrophilic aromatic substitution because this reactivity was found to be independent of oxygen and acid mediated. On the contrary, the dimerization was dependent on the presence of oxygen and thus likely involves radical intermediates. Thus this report overall displays different accessible reaction pathways for BTO that can be exploited for the production of BTO-derived compounds.
中文翻译:

生物基化学品:1,2,4-苯撑三醇,选择性氘化和二聚为双功能芳族化合物
源自碳水化合物衍生的平台化学物质5-羟甲基甲基糠醛(HMF)的1,2,4-苯三醇(BTO)是合成各种生物基芳香族产品的有趣起点。但是,BTO在温和条件下很容易发生二聚化和其他反应,这使分析和分离变得困难。为了控制和利用BTO的反应性来生产生物基构件,需要更好地了解其反应性。在这里发现特定的BTO芳香族C–H键对与D 2的氘交换具有反应性O在室温下的酸性条件下表现出明显的O,可导致带有芳香环的BTO选择性形成,该芳香环包含一个或两个氘原子,第一个在五个位置,第二个在三个位置。通过暴露于空气,表明BTO形成5,5'-连接的BTO二聚体[1,1'-联苯] -2,2',4,4',5,5'-己醇(1)含有二聚物结构2',4,4',5'-四羟基-[1,1'-联苯] -2,5-二酮的羟基醌(2)。此外,缩合二聚体二苯并[ b,d ]呋喃-2,3,7,8-四醇(3)可以相对容易地访问。这些对称和不对称的多功能二聚体的受控形成说明了BTO转化为有价值的生物基芳香族化合物的多种可能性。氘交换归因于亲电子芳族取代,因为发现该反应性独立于氧和酸介导。相反,二聚反应取决于氧的存在,因此可能涉及自由基中间体。因此,本报告总体上显示了可用于生产BTO衍生化合物的BTO的不同可及反应途径。
更新日期:2018-11-09
中文翻译:

生物基化学品:1,2,4-苯撑三醇,选择性氘化和二聚为双功能芳族化合物
源自碳水化合物衍生的平台化学物质5-羟甲基甲基糠醛(HMF)的1,2,4-苯三醇(BTO)是合成各种生物基芳香族产品的有趣起点。但是,BTO在温和条件下很容易发生二聚化和其他反应,这使分析和分离变得困难。为了控制和利用BTO的反应性来生产生物基构件,需要更好地了解其反应性。在这里发现特定的BTO芳香族C–H键对与D 2的氘交换具有反应性O在室温下的酸性条件下表现出明显的O,可导致带有芳香环的BTO选择性形成,该芳香环包含一个或两个氘原子,第一个在五个位置,第二个在三个位置。通过暴露于空气,表明BTO形成5,5'-连接的BTO二聚体[1,1'-联苯] -2,2',4,4',5,5'-己醇(1)含有二聚物结构2',4,4',5'-四羟基-[1,1'-联苯] -2,5-二酮的羟基醌(2)。此外,缩合二聚体二苯并[ b,d ]呋喃-2,3,7,8-四醇(3)可以相对容易地访问。这些对称和不对称的多功能二聚体的受控形成说明了BTO转化为有价值的生物基芳香族化合物的多种可能性。氘交换归因于亲电子芳族取代,因为发现该反应性独立于氧和酸介导。相反,二聚反应取决于氧的存在,因此可能涉及自由基中间体。因此,本报告总体上显示了可用于生产BTO衍生化合物的BTO的不同可及反应途径。


























 京公网安备 11010802027423号
京公网安备 11010802027423号