当前位置:
X-MOL 学术
›
Chem. Asian J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Kinetic Resolution of Acylsilane Cyanohydrins via Organocatalytic Cycloetherification
Chemistry - An Asian Journal ( IF 4.1 ) Pub Date : 2018-11-27 , DOI: 10.1002/asia.201801600 Akira Matsumoto 1 , Keisuke Asano 1 , Seijiro Matsubara 1
Chemistry - An Asian Journal ( IF 4.1 ) Pub Date : 2018-11-27 , DOI: 10.1002/asia.201801600 Akira Matsumoto 1 , Keisuke Asano 1 , Seijiro Matsubara 1
Affiliation
|
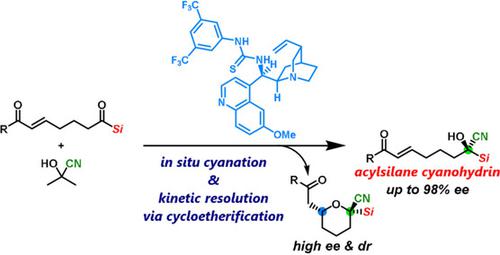
An asymmetric cyanation of acylsilanes involving the in‐situ formation of chiral acylsilane cyanohydrins followed by their kinetic resolution via organocatalytic cycloetherification is described. The highly enantio‐ and diastereoselective cycloetherification was crucial for achieving a high efficiency in the kinetic resolution. Consequently, acylsilane cyanohydrins containing a tetrasubstituted chiral carbon atom bearing silyl, cyano, and hydroxy groups were obtained in an enantioenriched form. This protocol therefore offers an efficient catalytic approach to optically active acylsilane cyanohydrins, which exhibit potential as chiral building blocks for the synthesis of pharmaceutically relevant chiral organosilanes.
中文翻译:

酰基硅烷氰醇通过有机催化环醚化的动力学拆分
描述了酰基硅烷的不对称氰化反应,涉及手性酰基硅烷氰醇的原位形成,然后通过有机催化环醚化进行动力学拆分。高度对映和非对映选择性的环醚化对于实现动力学拆分的高效率至关重要。因此,获得了具有对映体富集形式的含有带有甲硅烷基,氰基和羟基的四取代的手性碳原子的酰基硅烷氰醇。因此,该方案为旋光酰基硅烷氰醇提供了一种有效的催化方法,该化合物具有潜在的手性构建基,可用于合成药物相关的手性有机硅烷。
更新日期:2018-11-27
中文翻译:

酰基硅烷氰醇通过有机催化环醚化的动力学拆分
描述了酰基硅烷的不对称氰化反应,涉及手性酰基硅烷氰醇的原位形成,然后通过有机催化环醚化进行动力学拆分。高度对映和非对映选择性的环醚化对于实现动力学拆分的高效率至关重要。因此,获得了具有对映体富集形式的含有带有甲硅烷基,氰基和羟基的四取代的手性碳原子的酰基硅烷氰醇。因此,该方案为旋光酰基硅烷氰醇提供了一种有效的催化方法,该化合物具有潜在的手性构建基,可用于合成药物相关的手性有机硅烷。



























 京公网安备 11010802027423号
京公网安备 11010802027423号