当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Evaluated Site-Specific Rate Constants for Reaction of Isobutane with H and CH3: Shock Tube Experiments Combined with Bayesian Model Optimization
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2018-11-08 00:00:00 , DOI: 10.1021/acs.jpca.8b08781 Laura A. Mertens 1 , Iftikhar A. Awan 1 , David A. Sheen 1 , Jeffrey A. Manion 1
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2018-11-08 00:00:00 , DOI: 10.1021/acs.jpca.8b08781 Laura A. Mertens 1 , Iftikhar A. Awan 1 , David A. Sheen 1 , Jeffrey A. Manion 1
Affiliation
|
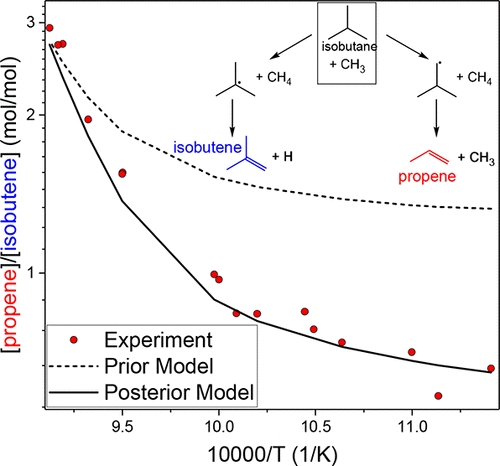
Evaluated site-specific rate constants for the reactions of isobutane with CH3 and H were determined in a combined analysis of new shock tube experiments and existing literature data. In our shock tube experiments, CH3 radicals, produced from the pyrolysis of di-tert-butylperoxide, and H atoms, produced from the pyrolysis of C2H5I, were reacted with dilute mixtures of isobutane in argon at 870–1130 K and 140–360 kPa, usually with a radical chain inhibitor. Propene and isobutene, measured with GC/FID and MS, were quantified as characteristic of H-abstraction from the primary and tertiary carbons, respectively. Using the method of uncertainty minimization using polynomial chaos expansions (MUM-PCE), a comprehensive Cantera kinetics model based on JetSurF 2.0 was optimized to our experiments and available literature data spanning ambient temperatures to 1327 K. Based on Bayes’ theorem, MUM-PCE constrains the kinetics model to the experimental data. The isobutane literature data used for optimization included both raw experimental data and reported branching and total rate measurements. Data for ethane were also included to better define the absolute rate constant for abstraction of H from primary carbons. For both H and CH3, the optimization increased the relative rate of tertiary to primary H-abstraction compared with existing estimates, especially at higher temperatures. We combine the present data for primary and tertiary sites with previous results from our group on 1-butane to derive site-specific rate constants for the reaction of H and CH3 with generic primary, secondary, and tertiary carbons suitable for a wide range of temperatures.
中文翻译:

异丁烷与H和CH 3反应的特定位置速率常数的评估:结合贝叶斯模型优化的冲击管实验
在新的冲击管实验和现有文献数据的组合分析中,确定了异丁烷与CH 3和H反应的估计的比位速率常数。在我们的激波管实验中,由二叔丁基过氧化物的热解产生的CH 3自由基和由C 2 H 5的热解产生的H原子I与异丁烷在氩气中的稀混合物在870-1130 K和140-360 kPa下反应,通常与自由基链抑制剂反应。用GC / FID和MS测量的丙烯和异丁烯分别被量化为从伯碳和叔碳中吸氢的特征。使用多项式混沌展开式的不确定性最小化方法(MUM-PCE),针对我们的实验和可用文献数据优化了基于JetSurF 2.0的全面Cantera动力学模型,其环境温度范围为1327K。基于贝叶斯定理,MUM-PCE将动力学模型约束到实验数据。用于优化的异丁烷文献数据既包括原始实验数据,也包括报告的分支和总速率测量结果。还包括乙烷数据,以更好地定义从伯碳中提取H的绝对速率常数。对于H和CH如图3所示,与现有估计相比,该优化增加了第三级与一级H提取的相对速率,尤其是在较高温度下。我们将当前一级和三级位点的数据与我们小组在1-丁烷上的先前结果相结合,以得出H和CH 3与适用于广泛范围的通用一级,二级和三级碳的反应的位点速率常数。温度。
更新日期:2018-11-08
中文翻译:

异丁烷与H和CH 3反应的特定位置速率常数的评估:结合贝叶斯模型优化的冲击管实验
在新的冲击管实验和现有文献数据的组合分析中,确定了异丁烷与CH 3和H反应的估计的比位速率常数。在我们的激波管实验中,由二叔丁基过氧化物的热解产生的CH 3自由基和由C 2 H 5的热解产生的H原子I与异丁烷在氩气中的稀混合物在870-1130 K和140-360 kPa下反应,通常与自由基链抑制剂反应。用GC / FID和MS测量的丙烯和异丁烯分别被量化为从伯碳和叔碳中吸氢的特征。使用多项式混沌展开式的不确定性最小化方法(MUM-PCE),针对我们的实验和可用文献数据优化了基于JetSurF 2.0的全面Cantera动力学模型,其环境温度范围为1327K。基于贝叶斯定理,MUM-PCE将动力学模型约束到实验数据。用于优化的异丁烷文献数据既包括原始实验数据,也包括报告的分支和总速率测量结果。还包括乙烷数据,以更好地定义从伯碳中提取H的绝对速率常数。对于H和CH如图3所示,与现有估计相比,该优化增加了第三级与一级H提取的相对速率,尤其是在较高温度下。我们将当前一级和三级位点的数据与我们小组在1-丁烷上的先前结果相结合,以得出H和CH 3与适用于广泛范围的通用一级,二级和三级碳的反应的位点速率常数。温度。


























 京公网安备 11010802027423号
京公网安备 11010802027423号