Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2018-11-07 , DOI: 10.1016/j.bioorg.2018.10.061 Ewa Mironiuk-Puchalska , Włodzimierz Buchowicz , Piotr Grześkowiak , Patrycja Wińska , Monika Wielechowska , Olena Karatsai , Maria Jolanta Rędowicz , Maria Bretner , Mariola Koszytkowska-Stawińska
|
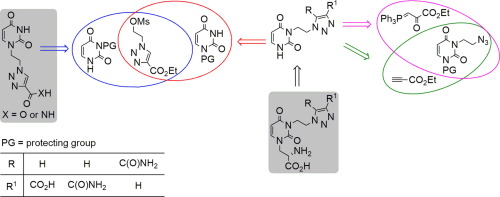
The 1H-1,2,3-triazole-originated derivatives of willardiine were obtained by: (i) construction of the 1H-1,2,3-triazole ring in 1,3-dipolar cycloaddition of the uracil-derived azides and the carboxylate-bearing alkynes or α-acylphosphorus ylide, or (ii) N-alkylation of the uracil derivative with the 1H-1,2,3-triazole-4-carboxylate-derived mesylate. The latter method offered: (i) reproducible results, (ii) a significant reduction of amounts of auxiliary materials, (iii) reduction in wastes and (iv) reduction in a number of manual operations required for obtaining the reaction product. Compound 6a exhibited significant binding affinity to hHS1S2I ligand-binding domain of GluR2 receptor (EC50 = 2.90 µM) and decreased viability of human astrocytoma MOG-G-CCM cells in higher extent than known AMPA antagonist GYKI 52466.
中文翻译:

β-uracilalanines的潜在生物电子等排衍生自ħ 1,2,3-三唑Ç -羧酸
1 H -1,2,3-三唑起源的柳丁碱衍生物可通过以下方法获得:(i)在尿嘧啶衍生的叠氮化物的1,3-偶极环加成中构建1 H -1,2,3-三唑环;和(ii)尿嘧啶衍生物与1 H -1,2,3-3-三唑-4-羧酸酯衍生的甲磺酸酯的尿烷衍生物的N-烷基化。后一种方法提供:(i)可重现的结果,(ii)辅助材料的量大大减少,(iii)废物减少,(iv)减少获得反应产物所需的许多手动操作。化合物6a对GluR2受体的hHS1S2I配体结合结构域表现出显着的结合亲和力(EC 50 = 2.90 µM),并比已知的AMPA拮抗剂GYKI 52466更高程度地降低了人类星形细胞瘤MOG-G-CCM细胞的活力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号