当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Theoretical and Kinetic Properties of OH Radical-Initiated Oxidation of Galaxolide in the Atmosphere
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2018-10-30 00:00:00 , DOI: 10.1021/acs.jpca.8b07456 Yunfeng Li 1 , Yanhui Sun 2 , Qingzhu Zhang 1
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2018-10-30 00:00:00 , DOI: 10.1021/acs.jpca.8b07456 Yunfeng Li 1 , Yanhui Sun 2 , Qingzhu Zhang 1
Affiliation
|
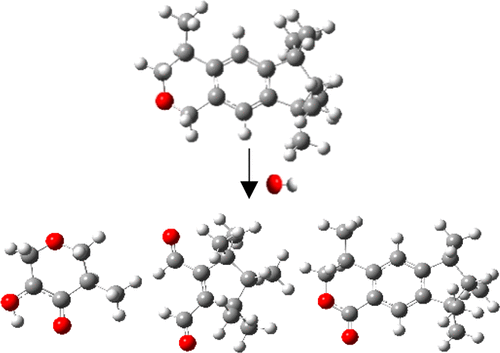
Galaxolide (HHCB), an important emerging contaminant, has attracted great environmental concern owing to its widespread occurrence and potential toxicity. In this study, the detailed multichannel mechanism of OH radical-initiated atmospheric degradation reactions of HHCB has been investigated by employing density functional theory (DFT). The reactants, transition states, intermediates, and products were optimized at the MPWB1K/6-31+G(d,p) level, and single-point energies were further refined at the MPWB1K/6-311+G(3df,2p) level of theory. The canonical variational transition-state (CVT) theory combined with the small curvature tunneling (SCT) was performed to evaluate the Arrhenius expressions and rate constants of key elementary reactions over a suitable range of 180–370 K. The thermodynamic and kinetic calculation results show that OH addition and hydrogen abstraction reactions are competitive pathways for HHCB. The dominant products in the presence of O2/NO are epoxide, dialdehyde, alcohol ketone, cyclolactone compounds, and HO2 radicals. At 298 K, the total rate constant of OH-initiated degradation of HHCB is 2.71 × 10–11 cm3 molecule–1 s–1. The atmospheric lifetime of HHCB determined by OH-initiated reactions is 10.09 h, which is in favor of the phenomenon of medium-range transport for HHCB in the atmosphere.
中文翻译:

大气中Galaxolide OH自由基引发的氧化的理论和动力学性质
Galaxolide(HHCB)是一种重要的新兴污染物,由于其广泛存在和潜在毒性而引起了极大的环境关注。在这项研究中,已通过使用密度泛函理论(DFT)研究了HHCB的OH自由基引发的大气降解反应的详细多通道机理。在MPWB1K / 6-31 + G(d,p)水平优化了反应物,过渡态,中间体和产物,并在MPWB1K / 6-311 + G(3df,2p)进一步优化了单点能量。理论水平。使用规范的变分过渡态(CVT)理论结合小曲率隧穿(SCT)来评估在180-370 K的适当范围内关键元素反应的Arrhenius表达式和速率常数。热力学和动力学计算结果表明,OH加成和夺氢反应是HHCB的竞争途径。O存在下的主导产品2 / NO是环氧化物,二醛,醇酮,环内酯化合物和HO 2基团。在298 K时,OH引发的HHCB降解的总速率常数为2.71×10 –11 cm 3分子–1 s –1。由OH引发的反应确定的HHCB的大气寿命为10.09 h,这有利于HHCB在大气中的中程迁移现象。
更新日期:2018-10-30
中文翻译:

大气中Galaxolide OH自由基引发的氧化的理论和动力学性质
Galaxolide(HHCB)是一种重要的新兴污染物,由于其广泛存在和潜在毒性而引起了极大的环境关注。在这项研究中,已通过使用密度泛函理论(DFT)研究了HHCB的OH自由基引发的大气降解反应的详细多通道机理。在MPWB1K / 6-31 + G(d,p)水平优化了反应物,过渡态,中间体和产物,并在MPWB1K / 6-311 + G(3df,2p)进一步优化了单点能量。理论水平。使用规范的变分过渡态(CVT)理论结合小曲率隧穿(SCT)来评估在180-370 K的适当范围内关键元素反应的Arrhenius表达式和速率常数。热力学和动力学计算结果表明,OH加成和夺氢反应是HHCB的竞争途径。O存在下的主导产品2 / NO是环氧化物,二醛,醇酮,环内酯化合物和HO 2基团。在298 K时,OH引发的HHCB降解的总速率常数为2.71×10 –11 cm 3分子–1 s –1。由OH引发的反应确定的HHCB的大气寿命为10.09 h,这有利于HHCB在大气中的中程迁移现象。

























 京公网安备 11010802027423号
京公网安备 11010802027423号