当前位置:
X-MOL 学术
›
Chem. Phys. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Dependence of NMR Chemical Shifts upon CH Bond Lengths of a Methyl Group Involved in a Tetrel Bond
Chemical Physics Letters ( IF 2.8 ) Pub Date : 2018-10-30 , DOI: 10.1016/j.cplett.2018.10.069 Steve Scheiner
中文翻译:

NMR化学位移对与Tetrel键有关的甲基的CH键长度的依赖性
更新日期:2018-10-31
Chemical Physics Letters ( IF 2.8 ) Pub Date : 2018-10-30 , DOI: 10.1016/j.cplett.2018.10.069 Steve Scheiner
|
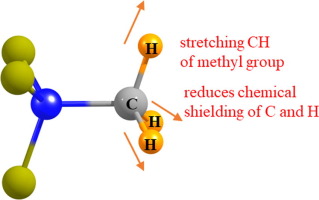
Four different Lewis acids that might participate in a tetrel bond with a nucleophile (SEt2Me+, NMe4+, SMe2, NMe3) are examined. The NMR chemical shifts of the methyl C and H atoms are calculated as the CH bond lengths are systematically stretched and contracted, in the absence of a base. The C shielding diminishes by roughly 2 ppm for a stretch of 0.01 Å, while that of H drops by only 0.3 ppm. The deshieldings caused purely by the bond length changes are far too small to account for the amounts that are computed when the nucleophile is actually present.
中文翻译:

NMR化学位移对与Tetrel键有关的甲基的CH键长度的依赖性
考察了可能与亲核体的锡et键结合的四种不同的路易斯酸(SEt 2 Me +,NMe 4 +,SMe 2,NMe 3)。甲基C和H原子的NMR化学位移是在不存在碱的情况下,通过系统地拉伸和收缩CH键长度来计算的。延伸0.01Å时,C屏蔽层大约减少2 ppm,而H的屏蔽层仅下降0.3 ppm。纯粹由键长变化引起的去屏蔽作用太小,不足以说明亲核试剂实际存在时的计算量。



























 京公网安备 11010802027423号
京公网安备 11010802027423号