Biochimie ( IF 3.9 ) Pub Date : 2018-10-31 , DOI: 10.1016/j.biochi.2018.10.020 Tetsuya Masuda , Kyohei Okubo , Kazuki Murata , Bunzo Mikami , Michihiro Sugahara , Mamoru Suzuki , Piero Andrea Temussi , Fumito Tani
|
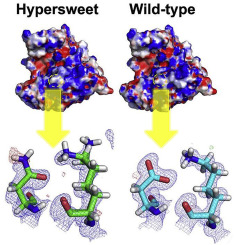
One of the sweetest proteins found in tropical fruits (with a threshold of 50 nM), thaumatin, is also used commercially as a sweetener. Our previous study successfully produced the sweetest thaumatin mutant (D21N), designated hyper-sweet thaumatin, which decreases the sweetness threshold to 31 nM. To investigate why the D21N mutant is sweeter than wild-type thaumatin, we compared the structure of the D21N mutant solved at a subatomic resolution of 0.93 Å with that of wild-type thaumatin determined at 0.90 Å. Although the overall structure of the D21N mutant resembles that of wild-type thaumatin, our subatomic resolution analysis successfully assigned and discriminated the detailed atomic positions of side-chains at position 21. The relative B-factor value of the side-chain at position 21 in the D21N mutant was higher than that of wild-type thaumatin, hinting at a greater flexibility of side-chain at 21 in the hyper-sweet D21N mutant. Furthermore, alternative conformations of Lys19, which is hydrogen-bonded to Asp21 in wild-type, were found only in the D21N mutant. Subatomic resolution analysis revealed that flexible conformations at the sites adjacent to positions 19 and 21 play a crucial role in enhancing sweet potency and may serve to enhance the complementarity of electrostatic potentials for interaction with the sweet taste receptor.
中文翻译:

超甜thaumatin D21N突变体的亚原子结构揭示了柔性构象对提高甜度的重要性
热带水果(阈值为50 nM)中发现的最甜的蛋白质之一thaumatin,也已在商业上用作甜味剂。我们之前的研究成功产生了最甜的thaumatin突变体(D21N),称为超甜thaumatin,将甜度阈值降低到31 nM。为了研究为什么D21N突变体比野生型thaumatin甜,我们比较了亚原子分辨率为0.93Å的D21N突变体的结构与0.90Å的野生型thaumatin的结构。虽然D21N的整体结构的突变体类似于野生型奇异果甜蛋白,我们的亚原子分辨率分析成功在位置21的相对分配和鉴别侧链的详细原子位置乙D21N突变体中第21位的侧链的α-因子值高于野生型thaumatin的值,这表明超甜D21N突变体中第21位的侧链具有更大的柔韧性。此外,仅在D21N突变体中发现了与野生型氢键合至Asp21的Lys19的替代构象。亚原子分辨率分析表明,与位置19和21相邻的位点上的柔性构象在增强甜味力中起着至关重要的作用,并且可能起到增强静电势与甜味受体相互作用的互补性的作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号