Biochimie ( IF 3.9 ) Pub Date : 2018-10-29 , DOI: 10.1016/j.biochi.2018.10.017 Konstantin M. Boyko , Timur N. Baymukhametov , Yury M. Chesnokov , Michael Hons , Sofya V. Lushchekina , Petr V. Konarev , Alexey V. Lipkin , Alexandre L. Vasiliev , Patrick Masson , Vladimir O. Popov , Michail V. Kovalchuk
|
Human plasma butyrylcholinesterase (BChE) is an endogenous bioscavenger that hydrolyzes numerous medicamentous and poisonous esters and scavenges potent organophosphorus nerve agents. BChE is thus a marker for the diagnosis of OP poisoning. It is also considered a therapeutic target against Alzheimer's disease. Although the X-ray structure of a partially deglycosylated monomer of human BChE was solved 15 years ago, all attempts to determine the 3D structure of the natural full-length glycosylated tetrameric human BChE have been unsuccessful so far.
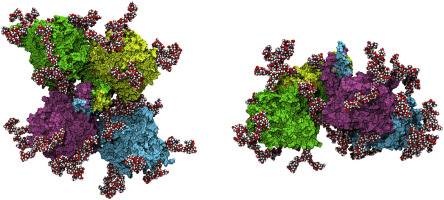
Here, a combination of three complementary structural methods—single-particle cryo-electron microscopy, molecular dynamics and small-angle X-ray scattering—were implemented to elucidate the overall structural and spatial organization of the natural tetrameric human plasma BChE. A 7.6 Å cryoEM map clearly shows the major features of the enzyme: a dimer of dimers with a nonplanar monomer arrangement, in which the interconnecting super helix complex PRAD-(WAT)4-peptide C-terminal tail is located in the center of the tetramer, nearly perpendicular to its plane, and is plunged deep between the four subunits. Molecular dynamics simulations allowed optimization of the geometry of the molecule and reconstruction of the structural features invisible in the cryoEM density, i.e., glycan chains and glycan interdimer contact areas, as well as intermonomer disulfide bridges at the C-terminal tail. Finally, SAXS data were used to confirm the consistency of the obtained model with the experimental data. The tetramer organization of BChE is unique in that the four subunits are joined at their C-termini through noncovalent contacts with a short polyproline-rich peptide. This tetramer structure could serve as a model for the design of highly stable glycosylated tetramers.
中文翻译:

cryoEM,SAXS和MD揭示的人丁酰胆碱酯酶天然四聚体形式的3D结构
人血浆丁酰胆碱酯酶(BChE)是一种内源性生物清除剂,它水解多种药物和有毒的酯类,并清除有效的有机磷神经毒剂。因此,BChE是诊断OP中毒的标志物。它也被认为是对抗阿尔茨海默氏病的治疗靶标。尽管人类BChE的部分去糖基化单体的X射线结构在15年前就已解决,但到目前为止,确定天然全长糖基化四聚体人类BChE的3D结构的所有尝试均未成功。
在这里,结合了三种互补的结构方法-单粒子冷冻电子显微镜,分子动力学和小角度X射线散射-阐明了天然四聚体人类血浆BChE的总体结构和空间组织。7.6ÅcryoEM图谱清楚地显示了该酶的主要特征:具有非平面单体排列的二聚体的二聚体,其中相互连接的超螺旋复合物PRAD-(WAT)4-肽的C-末端尾巴位于四聚体的中心,几乎垂直于其平面,并深陷于四个亚基之间。分子动力学模拟可以优化分子的几何形状,并重建cryoEM密度中不可见的结构特征,即聚糖链和聚糖互二聚体接触区域,以及C末端尾部的单体二硫键。最后,使用SAXS数据来确认所获得模型与实验数据的一致性。BChE的四聚体组织是独特的,因为四个亚基通过与富含脯氨酸的短肽的非共价接触在其C末端连接。该四聚体结构可以用作设计高度稳定的糖基化四聚体的模型。



























 京公网安备 11010802027423号
京公网安备 11010802027423号