当前位置:
X-MOL 学术
›
J. Proteome Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Global Identification of Protein Complexes within the Membrane Proteome of Arabidopsis Roots Using a SEC-MS Approach.
Journal of Proteome Research ( IF 4.4 ) Pub Date : 2018-11-13 , DOI: 10.1021/acs.jproteome.8b00382 Max Gilbert 1 , Waltraud X Schulze 1
Journal of Proteome Research ( IF 4.4 ) Pub Date : 2018-11-13 , DOI: 10.1021/acs.jproteome.8b00382 Max Gilbert 1 , Waltraud X Schulze 1
Affiliation
|
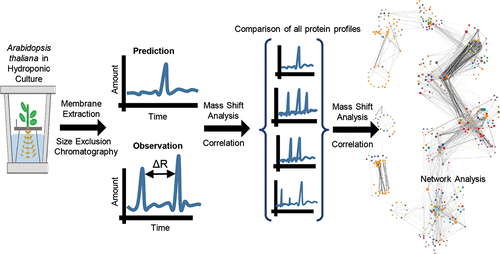
Biological processes consist of several consecutive and interacting steps as, for example, in signal transduction cascades or metabolic reaction chains. These processes are regulated by protein-protein interactions and the formation of larger protein complexes, which also occur within biological membranes. To gain a large-scale overview of complex-forming proteins and the composition of such complexes within the cellular membranes of Arabidopsis roots, we use the combination of size-exclusion chromatography and mass spectrometry. First, we identified complex-forming proteins by a retention shift analysis relative to expected retention times of monomeric proteins during size-exclusion chromatography. In a second step we predicted complex composition through pairwise correlation of elution profiles. As result we present an interactome of 963 proteins within cellular membranes of Arabidopsis roots. Identification of complex-forming proteins was highly robust between two independently grown root proteomes. The protein complex composition derived from pairwise correlations of coeluting proteins reproducibly identified stable protein complexes (ribosomes, proteasome, mitochondrial respiratory chain supercomplexes) but showed higher variance between replicates regarding transient interactions (e.g., interactions with kinases) within membrane protein complexes.
中文翻译:

使用SEC-MS方法对拟南芥根的膜蛋白质组中的蛋白质复合物进行全局鉴定。
生物过程包括几个连续且相互作用的步骤,例如在信号转导级联或代谢反应链中。这些过程受蛋白质-蛋白质相互作用和较大蛋白质复合物形成的调节,这些蛋白质复合物也发生在生物膜内。为了获得大规模的复合物形成蛋白的概述以及拟南芥根细胞膜中此类复合物的组成,我们使用了体积排阻色谱法和质谱法的结合。首先,我们通过相对于单体排阻色谱法预期的单体蛋白质保留时间的保留偏移分析,鉴定了形成复合物的蛋白质。在第二步中,我们通过洗脱曲线的成对相关性预测了复杂的成分。结果,我们提出了拟南芥根细胞膜内963种蛋白质的相互作用组。在两个独立生长的根蛋白质组之间鉴定形成复合物的蛋白质是高度可靠的。从共洗脱蛋白的成对相关性得出的蛋白复合物组成可重复地鉴定出稳定的蛋白复合物(核糖体,蛋白酶体,线粒体呼吸链超复合物),但在膜蛋白复合物中关于瞬时相互作用(例如与激酶的相互作用)的重复之间显示出更高的差异。
更新日期:2018-11-13
中文翻译:

使用SEC-MS方法对拟南芥根的膜蛋白质组中的蛋白质复合物进行全局鉴定。
生物过程包括几个连续且相互作用的步骤,例如在信号转导级联或代谢反应链中。这些过程受蛋白质-蛋白质相互作用和较大蛋白质复合物形成的调节,这些蛋白质复合物也发生在生物膜内。为了获得大规模的复合物形成蛋白的概述以及拟南芥根细胞膜中此类复合物的组成,我们使用了体积排阻色谱法和质谱法的结合。首先,我们通过相对于单体排阻色谱法预期的单体蛋白质保留时间的保留偏移分析,鉴定了形成复合物的蛋白质。在第二步中,我们通过洗脱曲线的成对相关性预测了复杂的成分。结果,我们提出了拟南芥根细胞膜内963种蛋白质的相互作用组。在两个独立生长的根蛋白质组之间鉴定形成复合物的蛋白质是高度可靠的。从共洗脱蛋白的成对相关性得出的蛋白复合物组成可重复地鉴定出稳定的蛋白复合物(核糖体,蛋白酶体,线粒体呼吸链超复合物),但在膜蛋白复合物中关于瞬时相互作用(例如与激酶的相互作用)的重复之间显示出更高的差异。



























 京公网安备 11010802027423号
京公网安备 11010802027423号