当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Inhibition of Human Topoisomerase II by N,N,N‐Trimethylethanammonium Iodide Alkylcarbazole Derivatives
ChemMedChem ( IF 3.4 ) Pub Date : 2018-11-30 , DOI: 10.1002/cmdc.201800546 Carmela Saturnino 1 , Anna Caruso 2 , Domenico Iacopetta 2 , Camillo Rosano 3 , Jessica Ceramella 2 , Noemi Muià 2 , Annaluisa Mariconda 4 , Maria Grazia Bonomo 1 , Marco Ponassi 3 , Giuseppe Rosace 4 , Maria Stefania Sinicropi 2 , Pasquale Longo 5
ChemMedChem ( IF 3.4 ) Pub Date : 2018-11-30 , DOI: 10.1002/cmdc.201800546 Carmela Saturnino 1 , Anna Caruso 2 , Domenico Iacopetta 2 , Camillo Rosano 3 , Jessica Ceramella 2 , Noemi Muià 2 , Annaluisa Mariconda 4 , Maria Grazia Bonomo 1 , Marco Ponassi 3 , Giuseppe Rosace 4 , Maria Stefania Sinicropi 2 , Pasquale Longo 5
Affiliation
|
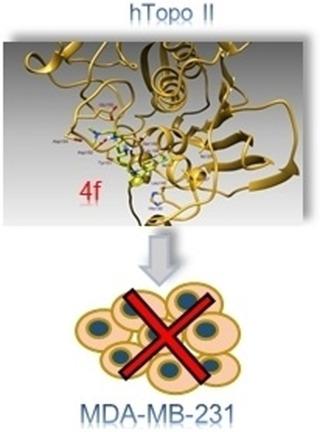
Chemotherapy is used for the treatment of all stages of breast cancer, including the metastatic stage of the disease. Treatment regimens are generally tailored for each patient′s particular situation. However, chemotherapeutic agents are the leading cause of serious drug‐related adverse effects; moreover, drug resistance often occurs. In this study, we designed and synthesized a new series of N‐alkylcarbazoles derived from ellipticine, an alkaloid with a carbazole skeleton initially used in the treatment of metastatic breast cancer and later dismissed because of poor aqueous solubility and severe side effects. After evaluating the binding modes of our class of newly synthesized compounds with human topoisomerase II (hTopo II), we performed hTopo II decatenation assays, identifying compound 4 f (2‐(4‐((3‐chloro‐9H‐carbazol‐9‐yl)pentyl)piperazin‐1‐yl)‐N,N,N‐trimethylethanammonium iodide) as a good inhibitor. Moreover, 4 f and 4 g (2‐(4‐((3‐chloro‐9H‐carbazol‐9‐yl)hexyl)piperazin‐1‐yl)‐N,N,N‐trimethylethanammonium iodide) showed a good anti‐proliferative activity toward breast cancer cells, causing apoptosis by activation of the caspase pathway. Interestingly, the activity of these two compounds on triple‐negative MDA‐MB‐231 cells, which tend to be highly metastatic and aggressive, is strictly connected to the observed inhibition of hTopo II.
中文翻译:

N,N,N-碘化三碘甲烷烷基咔唑衍生物对人拓扑异构酶II的抑制作用
化学疗法用于治疗乳腺癌的所有阶段,包括疾病的转移阶段。通常针对每个患者的特定情况量身定制治疗方案。然而,化学治疗药物是严重的药物相关不良反应的主要原因。此外,经常发生耐药性。在这项研究中,我们设计和合成了一系列新的由玫瑰树碱衍生的N烷基咔唑,玫瑰碱是一种具有咔唑骨架的生物碱,最初用于治疗转移性乳腺癌,后来由于水溶性差和严重的副作用而被淘汰。在评估了我们这类新合成的化合物与人类拓扑异构酶II(hTopo II)的结合模式后,我们进行了hTopo II脱级分析,鉴定了化合物4 f(2-(4-(((3-氯-9 H-咔唑-9-基)戊基)哌嗪-1-基)-N,N,N-三甲基碘化碘化铵)是一种良好的抑制剂。另外,图4f和4克(2-(4 - ((3-氯-9- ħ咔唑-9-基)己基)哌嗪-1-基) - ñ,Ñ,Ñ -trimethylethanammonium碘化物)显示出良好的抗对乳腺癌细胞的增殖活性,通过激活caspase途径引起细胞凋亡。有趣的是,这两种化合物在三阴性MDA-MB-231细胞上的活性通常高度转移和具有侵略性,与观察到的hTopo II抑制作用密切相关。
更新日期:2018-11-30
中文翻译:

N,N,N-碘化三碘甲烷烷基咔唑衍生物对人拓扑异构酶II的抑制作用
化学疗法用于治疗乳腺癌的所有阶段,包括疾病的转移阶段。通常针对每个患者的特定情况量身定制治疗方案。然而,化学治疗药物是严重的药物相关不良反应的主要原因。此外,经常发生耐药性。在这项研究中,我们设计和合成了一系列新的由玫瑰树碱衍生的N烷基咔唑,玫瑰碱是一种具有咔唑骨架的生物碱,最初用于治疗转移性乳腺癌,后来由于水溶性差和严重的副作用而被淘汰。在评估了我们这类新合成的化合物与人类拓扑异构酶II(hTopo II)的结合模式后,我们进行了hTopo II脱级分析,鉴定了化合物4 f(2-(4-(((3-氯-9 H-咔唑-9-基)戊基)哌嗪-1-基)-N,N,N-三甲基碘化碘化铵)是一种良好的抑制剂。另外,图4f和4克(2-(4 - ((3-氯-9- ħ咔唑-9-基)己基)哌嗪-1-基) - ñ,Ñ,Ñ -trimethylethanammonium碘化物)显示出良好的抗对乳腺癌细胞的增殖活性,通过激活caspase途径引起细胞凋亡。有趣的是,这两种化合物在三阴性MDA-MB-231细胞上的活性通常高度转移和具有侵略性,与观察到的hTopo II抑制作用密切相关。


























 京公网安备 11010802027423号
京公网安备 11010802027423号