Journal of the Mechanical Behavior of Biomedical Materials ( IF 3.9 ) Pub Date : 2018-10-21 , DOI: 10.1016/j.jmbbm.2018.10.005 Kazuaki Nagayama 1 , Keiichi Uchida 1 , Akiko Sato 1
|
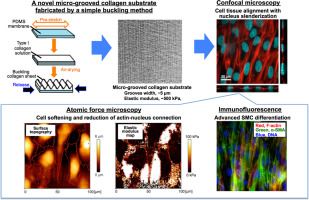
Vascular smooth muscle cells (SMCs) actively remodel arterial walls through biomechanical signals and dedifferentiate from the contractile to the synthetic state under pathological conditions. It is important to determine the differentiation mechanism of SMCs to understand their pathophysiology in disease. Previously, we found that the F-actin cytoskeleton in dedifferentiated SMCs on dishes was firmly connected to the nucleus, and that internal mechanical signals in SMCs are transmitted directly to the nucleus, indicating that nuclear-cytoskeletal interactions could be associated with SMC differentiation. However, mechanical environments in vivo are quite different from those of cultured cells: SMCs in vivo show an elongated shape and form a tissue that aligns with the circumferential direction of the walls. Thus, in the present study, we established a simple technique to fabricate a novel micro-grooved native collagen substrate that mimics the elongated cell shapes and alignment observed in vivo. The substrates had “wavy wrinkle” grooves with a width of ~5 µm and a Young's modulus of ~500 kPa, which were quite similar to those of the elastic lamina in vascular tissues. Using confocal microscopy image-based analysis, and nano-indentation imaging with atomic force microscopy, we found that SMCs on the micro-grooved collagen formed significant cell tissue arrangement, and changed their nuclear morphology to a “slim ellipsoid” in response to the force-reduction caused by F-actin remodeling, which consequently improved SMC differentiation. These findings indicated that this type of intracellular force-reduction around the nucleus has a crucial effect on SMC differentiation. Our micro-grooved collagen substrate is a powerful tool to investigate the mechanism of vascular SMC mechanotransduction.
中文翻译:

一种通过细胞组织排列和细胞核重塑诱导血管平滑肌分化的新型微沟槽胶原蛋白底物。
血管平滑肌细胞(SMC)通过生物力学信号主动重塑动脉壁,并在病理条件下从收缩状态向合成状态脱分化。确定SMC的分化机制以了解其在疾病中的病理生理学很重要。以前,我们发现碟子上去分化的SMC中的F-肌动蛋白细胞骨架与核牢固连接,并且SMC中的内部机械信号直接传递到核,表明核细胞骨架的相互作用可能与SMC分化有关。但是,体内的机械环境与培养细胞的机械环境大不相同:体内的SMC呈细长形状并形成与壁的圆周方向对齐的组织。因此,在本研究中,我们建立了一种简单的技术来制造新颖的微刻槽天然胶原蛋白底物,该底物模仿体内观察到的细长细胞形状和排列。基材具有“波浪状皱纹”凹槽,宽度为〜5 µm和〜500 kPa的杨氏模量,与血管组织中的弹性薄层非常相似。使用基于共聚焦显微镜的图像分析和原子力显微镜的纳米压痕成像,我们发现微槽胶原蛋白上的SMCs形成了明显的细胞组织排列,并根据该力将其核形态改变为“细长的椭圆体”。 -肌动蛋白重塑引起的β-还原减少,从而改善了SMC分化。这些发现表明,这种围绕细胞核的细胞内力减少对SMC分化具有关键作用。我们的微刻槽胶原蛋白底物是研究血管SMC机械转导机制的有力工具。



























 京公网安备 11010802027423号
京公网安备 11010802027423号