当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Lanthanide Photocatalysis
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2018-10-18 00:00:00 , DOI: 10.1021/acs.accounts.8b00336 Yusen Qiao 1 , Eric J. Schelter 1
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2018-10-18 00:00:00 , DOI: 10.1021/acs.accounts.8b00336 Yusen Qiao 1 , Eric J. Schelter 1
Affiliation
|
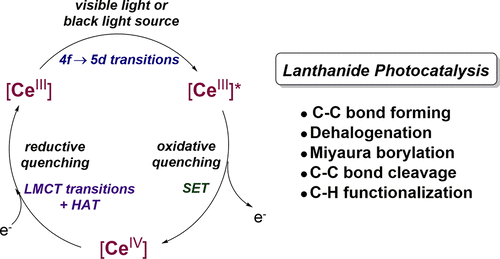
The use of earth-abundant, cheap, potent, and readily available lanthanide photocatalysts provides an opportunity to complement or even replace rare and precious metal photosensitizers. Moreover, lanthanide photosensitizers have been demonstrated for the generation of a variety of reactive species, including aryl radicals, alkyl radicals, and others, by single-electron-transfer (SET) and hydrogen atom transfer (HAT) pathways under mild reaction conditions. Some lanthanide photocatalysts have unprecedented reducing power from their photoexcited states, achieving the activation of challenging organic substrates that have not otherwise been activated by reported organic or transition-metal photosensitizers. In this Account, we describe our recent advances in the rational design and strategic application of lanthanide photo(redox)catalysis. Our research goals include understanding the photophysics of lanthanide luminophores and incorporating them into new photocatalysis. Among the lanthanides, we have focused on cerium because of the doublet to doublet 4f → 5d excitation and emission, which affords good conservation of energy without losses through spin-state changes, as well as a large natural abundance of that element. We have performed structural, spectroscopic, computational, and reactivity studies to demonstrate that luminescent Ce(III) guanidinate–amide complexes can mediate photocatalytic C(sp3)–C(sp3) bond forming reactions. Taking advantage of the strong reducing power of the cerium excited states and the cerium–halogen bond forming enthalpies, we determined that the reactive, excited-state cerium metalloradical abstracts chloride anion from benzyl chloride to generate the benzyl radical. To control and predict the photocatalytic reactivities, we have also performed photophysical and photochemical studies on a series of mixed-ligand Ce(III) guanidinate–amide and guanidinate–aryloxide complexes to establish structure–property relationship for Ce(III) photocatalysts. We discovered that the emission color is directly related to ligand type and rigidity of the coordination sphere and that the photoluminescent quantum yield is correlated to variation in steric encumbrance around the cerium centers. The low excited-state reduction potentials (E1/2* ≈ −2.1 to −2.9 V versus Cp2Fe0/+) and relatively fast quenching rates (kq ≈ 107 M–1 s–1) toward aryl halides enabled the Ce(III) guanidinate–amide complexes to participate in photocatalytic C(sp2)–C(sp2) bond forming reactions through either inner-sphere or outer-sphere SET processes. We have also reported a simple, potent, and air-stable ultraviolet A photoreductant, the hexachlorocerate(III) anion ([CeIIICl6]3–). This complex is a potent photoreductant (E1/2* ≈ −3.45 V versus Cp2Fe0/+) and exhibits a fast quenching kinetics (kq ≈ 109–1010 M–1 s–1) toward organohalogens. The [CeIVCl6]2− redox partner can also act as a potent photo-oxidant though a (presumably) long-lived chloride-to-cerium(IV) charge transfer excited state (ε = ∼6000 M–1 cm–1), that was used to turnover photocatalytic dechlorination and Miyuara borylation reactions. With [CeIIICl6]3–, we achieved efficient photoinduced dehalogenation and borylation of unactivated aryl chlorides with broad substrate scope, through formally two-photon cycles where both Ce(III) and Ce(IV) act as photocatalysts. Lanthanide photoredox catalysis is now being applied in several contexts for reactions including photocatalytic dehydrogenation of amines, alkoxy-radical-mediated C–C bond cleavage and amination of alkanols, and C–H activation of alkanes. Overall, simple and potent lanthanide photocatalysis is expected to find practical applications in organic synthesis, pharmaceutical development, and small molecule activation.
中文翻译:

镧系元素光催化
使用富含地球,廉价,有力且易于获得的镧系元素光催化剂提供了补充甚至替代稀有和贵金属光敏剂的机会。此外,已证明镧系光敏剂可在温和的反应条件下通过单电子转移(SET)和氢原子转移(HAT)途径生成各种反应性物种,包括芳基,烷基和其他自由基。一些镧系光催化剂从其光激发态开始具有空前的还原能力,从而实现了具有挑战性的有机底物的活化,而这些底物还没有被报道的有机或过渡金属光敏剂活化。在此报告中,我们描述了我们在镧系光(氧化还原)催化的合理设计和战略应用方面的最新进展。我们的研究目标包括了解镧系发光体的光物理性质并将其纳入新的光催化作用。在镧系元素中,我们将注意力集中在铈上,是因为从4t→5d的激发到发射的激发,这提供了良好的能量守恒,而不会因自旋态变化而损失,而且该元素的自然丰度也很高。我们已经进行了结构,光谱,计算和反应性研究,以证明发光的Ce(III)胍盐-酰胺配合物可以介导光催化C(sp 它提供了良好的能量守恒,不会因自旋态变化而损失,而且该元素的自然丰度也很高。我们已经进行了结构,光谱,计算和反应性研究,以证明发光的Ce(III)胍盐-酰胺配合物可以介导光催化C(sp 它提供了良好的能量守恒,不会因自旋态变化而损失,而且该元素的自然丰度也很高。我们已经进行了结构,光谱,计算和反应性研究,以证明发光的Ce(III)胍盐-酰胺配合物可以介导光催化C(sp3)–C(sp 3)形成键的反应。利用铈激发态和铈-卤素键形成焓的强大还原能力,我们确定了反应性,激发态的铈金属双金属从苄基氯中提取氯离子以生成苄基。为了控制和预测光催化反应活性,我们还对一系列混合配体Ce(III)胍盐-酰胺和胍基-芳基氧化物配合物进行了光物理和光化学研究,以建立Ce(III)光催化剂的结构-性质关系。我们发现,发射颜色与配体类型和配位球的刚度直接相关,并且光致发光量子产率与铈中心周围的空间负担变化有关。低激发态还原电位(È 1/2 * ≈-2.1 -2.9到与V的Cp 2的Fe 0 / +)和相对快的淬火速率(ķ q ≈10 7中号-1小号-1)朝向芳基卤使铈(III)胍基] -酰胺配合物通过内球或外球SET过程参与光催化的C(sp 2)–C(sp 2)键形成反应。我们还报告了一种简单,有效且空气稳定的紫外线A光还原剂,即六氯cerate(III)阴离子([Ce III Cl 6 ] 3–)。该络合物是有效的光还原剂(E 1/2* ≈-3.45 V对的Cp 2的Fe 0 / +)和显示出快速淬火动力学(ķ q ≈10 9 -10 10中号-1小号-1)朝向有机卤素。[Ce IV Cl 6 ] 2-氧化还原配偶体也可以作为有效的光氧化剂,尽管(假定)长寿命的氯-铈(IV)电荷转移激发态(ε=〜6000 M –1 cm – 1),用于周转光催化脱氯和Miyuara硼化反应。使用[Ce III Cl 6 ] 3–,我们通过正式的双光子循环(其中Ce(III)和Ce(IV)均充当光催化剂)实现了具有宽泛底物范围的未活化的芳基氯化物的有效光致脱卤和硼化。镧系元素的光氧化还原催化现在已用于多种反应中,包括胺的光催化脱氢,烷氧基-自由基介导的C–C键裂解和烷醇胺化以及烷烃的C–H活化。总体而言,简单有效的镧系元素光催化有望在有机合成,药物开发和小分子活化中找到实际应用。
更新日期:2018-10-18
中文翻译:

镧系元素光催化
使用富含地球,廉价,有力且易于获得的镧系元素光催化剂提供了补充甚至替代稀有和贵金属光敏剂的机会。此外,已证明镧系光敏剂可在温和的反应条件下通过单电子转移(SET)和氢原子转移(HAT)途径生成各种反应性物种,包括芳基,烷基和其他自由基。一些镧系光催化剂从其光激发态开始具有空前的还原能力,从而实现了具有挑战性的有机底物的活化,而这些底物还没有被报道的有机或过渡金属光敏剂活化。在此报告中,我们描述了我们在镧系光(氧化还原)催化的合理设计和战略应用方面的最新进展。我们的研究目标包括了解镧系发光体的光物理性质并将其纳入新的光催化作用。在镧系元素中,我们将注意力集中在铈上,是因为从4t→5d的激发到发射的激发,这提供了良好的能量守恒,而不会因自旋态变化而损失,而且该元素的自然丰度也很高。我们已经进行了结构,光谱,计算和反应性研究,以证明发光的Ce(III)胍盐-酰胺配合物可以介导光催化C(sp 它提供了良好的能量守恒,不会因自旋态变化而损失,而且该元素的自然丰度也很高。我们已经进行了结构,光谱,计算和反应性研究,以证明发光的Ce(III)胍盐-酰胺配合物可以介导光催化C(sp 它提供了良好的能量守恒,不会因自旋态变化而损失,而且该元素的自然丰度也很高。我们已经进行了结构,光谱,计算和反应性研究,以证明发光的Ce(III)胍盐-酰胺配合物可以介导光催化C(sp3)–C(sp 3)形成键的反应。利用铈激发态和铈-卤素键形成焓的强大还原能力,我们确定了反应性,激发态的铈金属双金属从苄基氯中提取氯离子以生成苄基。为了控制和预测光催化反应活性,我们还对一系列混合配体Ce(III)胍盐-酰胺和胍基-芳基氧化物配合物进行了光物理和光化学研究,以建立Ce(III)光催化剂的结构-性质关系。我们发现,发射颜色与配体类型和配位球的刚度直接相关,并且光致发光量子产率与铈中心周围的空间负担变化有关。低激发态还原电位(È 1/2 * ≈-2.1 -2.9到与V的Cp 2的Fe 0 / +)和相对快的淬火速率(ķ q ≈10 7中号-1小号-1)朝向芳基卤使铈(III)胍基] -酰胺配合物通过内球或外球SET过程参与光催化的C(sp 2)–C(sp 2)键形成反应。我们还报告了一种简单,有效且空气稳定的紫外线A光还原剂,即六氯cerate(III)阴离子([Ce III Cl 6 ] 3–)。该络合物是有效的光还原剂(E 1/2* ≈-3.45 V对的Cp 2的Fe 0 / +)和显示出快速淬火动力学(ķ q ≈10 9 -10 10中号-1小号-1)朝向有机卤素。[Ce IV Cl 6 ] 2-氧化还原配偶体也可以作为有效的光氧化剂,尽管(假定)长寿命的氯-铈(IV)电荷转移激发态(ε=〜6000 M –1 cm – 1),用于周转光催化脱氯和Miyuara硼化反应。使用[Ce III Cl 6 ] 3–,我们通过正式的双光子循环(其中Ce(III)和Ce(IV)均充当光催化剂)实现了具有宽泛底物范围的未活化的芳基氯化物的有效光致脱卤和硼化。镧系元素的光氧化还原催化现在已用于多种反应中,包括胺的光催化脱氢,烷氧基-自由基介导的C–C键裂解和烷醇胺化以及烷烃的C–H活化。总体而言,简单有效的镧系元素光催化有望在有机合成,药物开发和小分子活化中找到实际应用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号