当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Methane Hydration‐Shell Structure and Fragility
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-10-18 , DOI: 10.1002/anie.201809372 Xiangen Wu 1 , Wanjun Lu 2 , Louis M. Streacker 3 , Henry S. Ashbaugh 4 , Dor Ben‐Amotz 3
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-10-18 , DOI: 10.1002/anie.201809372 Xiangen Wu 1 , Wanjun Lu 2 , Louis M. Streacker 3 , Henry S. Ashbaugh 4 , Dor Ben‐Amotz 3
Affiliation
|
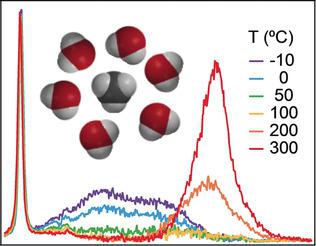
The influence of oily molecules on the structure of liquid water is a question of importance to biology and geology and many other fields. Previous experimental, theoretical, and simulation studies of methane in liquid water have reached widely conflicting conclusions regarding the structure of hydrophobic hydration‐shells. Herein we address this question by performing Raman hydration‐shell vibrational spectroscopic measurements of methane in liquid water from −10 °C to 300 °C (at 30 MPa, along a path that parallels the liquid‐vapor coexistence curve). We show that, near ambient temperatures, methane's hydration‐shell is slightly more tetrahedral than pure water. Moreover, the hydration‐shell undergoes a crossover to a more disordered structure above ca. 85 °C. Comparisons with the crossover temperature of aqueous methanol (and other alcohols) reveal the stabilizing influence of an alcohol OH head‐group on hydrophobic hydration‐shell fragility.
中文翻译:

甲烷水合壳结构和易碎性
油性分子对液态水结构的影响是生物学和地质学以及许多其他领域的重要问题。先前关于液态水中甲烷的实验,理论和模拟研究在疏水性水合壳的结构上得出了广泛矛盾的结论。在这里,我们通过对-10°C至300°C(在30 MPa下,沿着与液体-蒸汽共存曲线平行的路径)的液态水中的甲烷进行拉曼水化壳振动光谱测量,来解决这个问题。我们表明,在环境温度附近,甲烷的水合壳比纯水略多于四面体。此外,水化壳经历了一个转变,变成了一个高于约0的更无序的结构。85℃。
更新日期:2018-10-18
中文翻译:

甲烷水合壳结构和易碎性
油性分子对液态水结构的影响是生物学和地质学以及许多其他领域的重要问题。先前关于液态水中甲烷的实验,理论和模拟研究在疏水性水合壳的结构上得出了广泛矛盾的结论。在这里,我们通过对-10°C至300°C(在30 MPa下,沿着与液体-蒸汽共存曲线平行的路径)的液态水中的甲烷进行拉曼水化壳振动光谱测量,来解决这个问题。我们表明,在环境温度附近,甲烷的水合壳比纯水略多于四面体。此外,水化壳经历了一个转变,变成了一个高于约0的更无序的结构。85℃。


























 京公网安备 11010802027423号
京公网安备 11010802027423号