Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Codelivery of a cytotoxin and photosensitiser via a liposomal nanocarrier: a novel strategy for light-triggered cytosolic release†
Nanoscale ( IF 6.7 ) Pub Date : 2018-10-18 00:00:00 , DOI: 10.1039/c8nr04048f Elnaz Yaghini 1 , Ruggero Dondi , Karen J Edler , Marilena Loizidou , Alexander J MacRobert , Ian M Eggleston
Nanoscale ( IF 6.7 ) Pub Date : 2018-10-18 00:00:00 , DOI: 10.1039/c8nr04048f Elnaz Yaghini 1 , Ruggero Dondi , Karen J Edler , Marilena Loizidou , Alexander J MacRobert , Ian M Eggleston
Affiliation
|
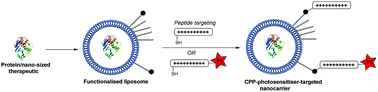
Endosomal entrapment is a key issue for the intracellular delivery of many nano-sized biotherapeutics to their cytosolic or nuclear targets. Photochemical internalisation (PCI) is a novel light-based solution that can be used to trigger the endosomal escape of a range of bioactive agents into the cytosol leading to improved efficacy in pre-clinical and clinical studies. PCI typically depends upon the endolysosomal colocalisation of the bioactive agent with a suitable photosensitiser that is administered separately. In this study we demonstrate that both these components may be combined for codelivery via a novel multifunctional liposomal nanocarrier, with a corresponding increase in the biological efficacy of the encapsulated agent. As proof of concept, we show here that the cytotoxicity of the 30 kDa protein toxin, saporin, in MC28 fibrosarcoma cells is significantly enhanced when delivered via a cell penetrating peptide (CPP)-modified liposome, with the CPP additionally functionalised with a photosensitiser that is targeted to endolysosomal membranes. This innovation opens the way for the efficient delivery of a range of biotherapeutics by the PCI approach, incorporating a clinically proven liposome delivery platform and using bioorthogonal ligation chemistries to append photosensitisers and peptides of choice.
中文翻译:

通过脂质体纳米载体共同递送细胞毒素和光敏剂:光触发胞质释放的新策略†
内体截留是许多纳米级生物治疗药物在细胞内递送至胞质或核靶点的关键问题。光化学内化(PCI)是一种新型的基于光的解决方案,可用于触发一系列生物活性剂从内体逃逸到细胞质中,从而提高临床前和临床研究的功效。PCI通常取决于生物活性剂与单独施用的合适光敏剂的内溶酶体共定位。在这项研究中,我们证明这两种成分可以通过新型多功能脂质体纳米载体组合进行共同递送,从而相应提高封装药物的生物功效。作为概念证明,我们在此表明,当通过细胞穿透肽 (CPP) 修饰的脂质体递送时,30 kDa 蛋白毒素皂草素在 MC28 纤维肉瘤细胞中的细胞毒性显着增强,并且 CPP 还用光敏剂进行了功能化,靶向内溶酶体膜。这项创新为通过 PCI 方法有效递送一系列生物治疗药物开辟了道路,结合了经过临床验证的脂质体递送平台,并使用生物正交连接化学来附加光敏剂和选择的肽。
更新日期:2018-10-18
中文翻译:

通过脂质体纳米载体共同递送细胞毒素和光敏剂:光触发胞质释放的新策略†
内体截留是许多纳米级生物治疗药物在细胞内递送至胞质或核靶点的关键问题。光化学内化(PCI)是一种新型的基于光的解决方案,可用于触发一系列生物活性剂从内体逃逸到细胞质中,从而提高临床前和临床研究的功效。PCI通常取决于生物活性剂与单独施用的合适光敏剂的内溶酶体共定位。在这项研究中,我们证明这两种成分可以通过新型多功能脂质体纳米载体组合进行共同递送,从而相应提高封装药物的生物功效。作为概念证明,我们在此表明,当通过细胞穿透肽 (CPP) 修饰的脂质体递送时,30 kDa 蛋白毒素皂草素在 MC28 纤维肉瘤细胞中的细胞毒性显着增强,并且 CPP 还用光敏剂进行了功能化,靶向内溶酶体膜。这项创新为通过 PCI 方法有效递送一系列生物治疗药物开辟了道路,结合了经过临床验证的脂质体递送平台,并使用生物正交连接化学来附加光敏剂和选择的肽。



























 京公网安备 11010802027423号
京公网安备 11010802027423号