Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Tumor-Microenvironment-Responsive Nanoconjugate for Synergistic Antivascular Activity and Phototherapy
ACS Nano ( IF 17.1 ) Pub Date : 2018-10-16 00:00:00 , DOI: 10.1021/acsnano.8b06478 Pingping Liang , Xiaoyu Huang , Ya Wang , Dapeng Chen , Changjin Ou , Qi Zhang , Jinjun Shao , Wei Huang 1 , Xiaochen Dong
ACS Nano ( IF 17.1 ) Pub Date : 2018-10-16 00:00:00 , DOI: 10.1021/acsnano.8b06478 Pingping Liang , Xiaoyu Huang , Ya Wang , Dapeng Chen , Changjin Ou , Qi Zhang , Jinjun Shao , Wei Huang 1 , Xiaochen Dong
Affiliation
|
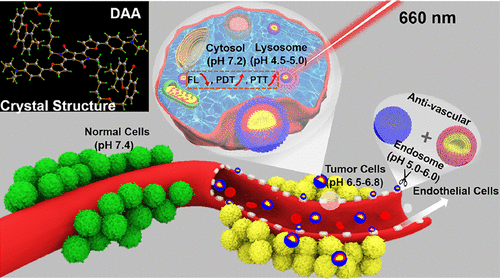
Insufficient oxygen supply (hypoxia), short half-life (<40 ns) of singlet oxygen, and up-regulation of the heat shock protein expression in solid tumors impede the photodynamic and photothermal therapeutic efficacy. Herein, a near-infrared carrier-free nanoconjugate direct-acting antiviral (DAA) with synergistic antivascular activity and pH-responsive photodynamic/photothermal behavior was designed and synthesized to improve cancer treatment efficacy. Obtained by the self-assembly approach, the biocompatible DAA nanoparticles (NPs) displayed amplifying pH-responsive photodynamic/photothermal performance in an acidic tumor microenvironment due to the protonation of diethylaminophenyl units. Most important, the antivascular agent 5,6-dimethylxanthenone-4-acetic acid, targeting the vascular endothelial growth factor, can be smartly released from the pro-drug DAA via ester bond hydrolysis at the subacid endocytosis organelles in the endothelial cells, which can effectively destroy the vascular region to prevent tumor proliferation and metastasis. Hence, DAA NPs can specifically target vascular endothelial cells and tumorous lysosomes with desired cellular damage properties in vitro. Therefore, the tumors can be ablated completely with no recurrence and side effects in vivo, which implies that DAA NPs provide a promising approach for cancer treatment via synergistic antivascular activity and photodynamic/photothermal therapy.
中文翻译:

具有协同抗血管活性和光疗作用的肿瘤微环境响应纳米偶联物
氧气供应不足(缺氧)、单线态氧的半衰期短(<40 ns)以及实体瘤中热休克蛋白表达的上调阻碍了光动力和光热治疗效果。在此,设计并合成了一种具有协同抗血管活性和 pH 响应光动力/光热行为的近红外无载体纳米偶联直接作用抗病毒药物 (DAA),以提高癌症治疗效果。通过自组装方法获得的生物相容性 DAA 纳米粒子 (NPs) 由于二乙氨基苯基单元的质子化,在酸性肿瘤微环境中显示出增强的 pH 响应光动力/光热性能。最重要的是,抗血管剂 5,6-二甲基呫吨酮-4-乙酸,靶向血管内皮生长因子,通过酯键水解在内皮细胞中的弱酸性内吞细胞器,可有效破坏血管区域,阻止肿瘤增殖和转移。因此,DAA NPs 可以在体外特异性靶向具有所需细胞损伤特性的血管内皮细胞和肿瘤溶酶体。因此,肿瘤可以在体内完全消融,无复发和副作用,这意味着 DAA NPs通过协同抗血管活性和光动力/光热疗法为癌症治疗提供了一种有前途的方法。
更新日期:2018-10-16
中文翻译:

具有协同抗血管活性和光疗作用的肿瘤微环境响应纳米偶联物
氧气供应不足(缺氧)、单线态氧的半衰期短(<40 ns)以及实体瘤中热休克蛋白表达的上调阻碍了光动力和光热治疗效果。在此,设计并合成了一种具有协同抗血管活性和 pH 响应光动力/光热行为的近红外无载体纳米偶联直接作用抗病毒药物 (DAA),以提高癌症治疗效果。通过自组装方法获得的生物相容性 DAA 纳米粒子 (NPs) 由于二乙氨基苯基单元的质子化,在酸性肿瘤微环境中显示出增强的 pH 响应光动力/光热性能。最重要的是,抗血管剂 5,6-二甲基呫吨酮-4-乙酸,靶向血管内皮生长因子,通过酯键水解在内皮细胞中的弱酸性内吞细胞器,可有效破坏血管区域,阻止肿瘤增殖和转移。因此,DAA NPs 可以在体外特异性靶向具有所需细胞损伤特性的血管内皮细胞和肿瘤溶酶体。因此,肿瘤可以在体内完全消融,无复发和副作用,这意味着 DAA NPs通过协同抗血管活性和光动力/光热疗法为癌症治疗提供了一种有前途的方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号