Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Turning a Luffa Protein into a Self-Assembled Biodegradable Nanoplatform for Multitargeted Cancer Therapy
ACS Nano ( IF 17.1 ) Pub Date : 2018-10-16 00:00:00 , DOI: 10.1021/acsnano.8b07079 Wangxiao He 1, 2 , Jin Yan 3 , Fang Sui 1 , Simeng Wang 1 , Xi Su 1 , Yiping Qu 1 , Qingchen Yang 2 , Hui Guo 1 , Meiju Ji 4 , Wuyuan Lu 5 , Yongping Shao 6 , Peng Hou 1
ACS Nano ( IF 17.1 ) Pub Date : 2018-10-16 00:00:00 , DOI: 10.1021/acsnano.8b07079 Wangxiao He 1, 2 , Jin Yan 3 , Fang Sui 1 , Simeng Wang 1 , Xi Su 1 , Yiping Qu 1 , Qingchen Yang 2 , Hui Guo 1 , Meiju Ji 4 , Wuyuan Lu 5 , Yongping Shao 6 , Peng Hou 1
Affiliation
|
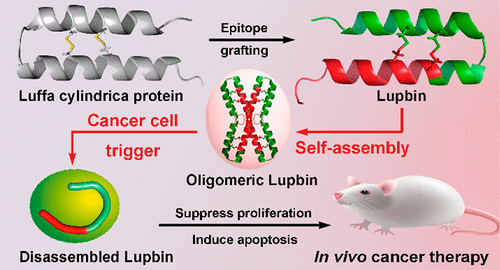
The peptide-derived self-assembly platform has attracted increasing attention for its great potential to develop into multitargeting nanomedicines as well as its inherent biocompatibility and biodegradability. However, their clinical application potentials are often compromised by low stability, weak membrane penetrating ability, and limited functions. Herein, inspired by a natural protein from the seeds of Luffa cylindrica, we engineered via epitope grafting and structure design a hybrid peptide-based nanoplatform, termed Lupbin, which is capable of self-assembling into a stable superstructure and concurrently targeting multiple protein–protein interactions (PPIs) located in cytoplasm and nuclei. We showed that Lupbin can efficiently penetrate cell membrane, escape from early endosome-dependent degradation, and subsequently disassemble into free monomers with wide distribution in cytosol and nucleus. Importantly, Lupbin abrogated tumor growth and metastasis through concurrent blockade of the Wnt/β-catenin signaling and reactivation of the p53 signaling, with a highly favorable in vivo biosafety profile. Our strategy expands the application of self-assembled nanomedicines into targeting intercellular PPIs, provides a potential nanoplatform with high stability for multitargeted cancer therapy, and likely reinvigorates the development of peptide-based therapeutics for the treatment of different human diseases including cancer.
中文翻译:

将丝瓜蛋白转变为可自组装的可生物降解的纳米平台,用于多目标癌症治疗
肽衍生的自组装平台因其发展为多靶点纳米药物的巨大潜力以及其固有的生物相容性和生物降解性而受到越来越多的关注。但是,它们的临床应用潜力常常因稳定性低,膜穿透力弱和功能受限而受到损害。在此,我们以Luffa cylindrica种子的天然蛋白质为灵感,通过抗原决定簇的嫁接和结构设计是基于杂合肽的纳米平台,称为Lupbin,它能够自组装成稳定的超结构,并同时靶向位于细胞质和细胞核中的多种蛋白质-蛋白质相互作用(PPI)。我们表明,卢宾滨可以有效地穿透细胞膜,摆脱早期的依赖内体的降解,并随后分解成在细胞质和细胞核中广泛分布的游离单体。重要的是,Lupbin通过同时阻断Wnt /β-catenin信号传导和p53信号传导的活化,从而消除了肿瘤的生长和转移,在体内具有非常好的优势生物安全性概况。我们的策略将自组装纳米药物的应用范围扩展到靶向细胞间PPI,为多靶点癌症治疗提供具有高稳定性的潜在纳米平台,并有可能振兴用于治疗包括癌症在内的各种人类疾病的基于肽的疗法的发展。
更新日期:2018-10-16
中文翻译:

将丝瓜蛋白转变为可自组装的可生物降解的纳米平台,用于多目标癌症治疗
肽衍生的自组装平台因其发展为多靶点纳米药物的巨大潜力以及其固有的生物相容性和生物降解性而受到越来越多的关注。但是,它们的临床应用潜力常常因稳定性低,膜穿透力弱和功能受限而受到损害。在此,我们以Luffa cylindrica种子的天然蛋白质为灵感,通过抗原决定簇的嫁接和结构设计是基于杂合肽的纳米平台,称为Lupbin,它能够自组装成稳定的超结构,并同时靶向位于细胞质和细胞核中的多种蛋白质-蛋白质相互作用(PPI)。我们表明,卢宾滨可以有效地穿透细胞膜,摆脱早期的依赖内体的降解,并随后分解成在细胞质和细胞核中广泛分布的游离单体。重要的是,Lupbin通过同时阻断Wnt /β-catenin信号传导和p53信号传导的活化,从而消除了肿瘤的生长和转移,在体内具有非常好的优势生物安全性概况。我们的策略将自组装纳米药物的应用范围扩展到靶向细胞间PPI,为多靶点癌症治疗提供具有高稳定性的潜在纳米平台,并有可能振兴用于治疗包括癌症在内的各种人类疾病的基于肽的疗法的发展。



























 京公网安备 11010802027423号
京公网安备 11010802027423号