Current Opinion in Chemical Engineering ( IF 6.6 ) Pub Date : 2018-10-16 , DOI: 10.1016/j.coche.2018.09.001 Sebastian Vogg , Thomas Müller-Späth , Massimo Morbidelli
|
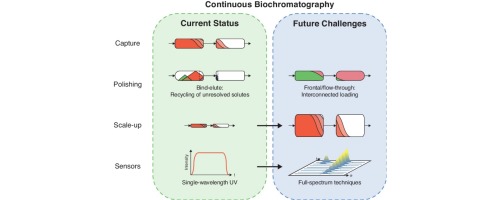
Continuous processing of biopharmaceuticals has seen increased interest in recent years due to more competition in the market as well as broader pipelines asking for more flexible and therefore intensified processes. For downstream processing, chromatography remains the separation process of choice due to its very high resolving power. While continuous counter-current processes have been implemented successfully in other sectors such as the petrochemical or small-molecule pharmaceutical industry, biopharmaceuticals bring new challenges to this field such as high mass transfer limitation due to their size. In this contribution, we present a concise review of continuous processes that have been developed or adapted to the needs of the biopharmaceutical sector and were successfully implemented at the lab scale. Furthermore, we highlight future challenges that need to be addressed both on the processing and the regulatory side. Resolving those will mature these processes to be employed at manufacturing scale. The discussed challenges include new operation modes as well as process scale-up and validation.
中文翻译:

连续生物色谱法的现状和未来挑战
近年来,由于市场上的竞争加剧以及要求更灵活,因此强化的流程的更广泛的管道,对生物制药的连续加工产生了越来越浓厚的兴趣。对于下游处理,由于其极高的分离能力,色谱法仍是选择的分离方法。尽管在石油化工或小分子制药行业等其他领域已经成功实施了连续逆流工艺,但生物制药给该领域带来了新的挑战,例如由于其体积大而限制了传质。在此贡献中,我们将简要介绍已开发或适应生物制药行业需求并已在实验室规模成功实施的连续过程。此外,我们着重指出在处理和监管方面都需要解决的未来挑战。解决这些问题将使这些过程趋于成熟,并可以在制造规模上采用。讨论的挑战包括新的操作模式以及工艺放大和验证。



























 京公网安备 11010802027423号
京公网安备 11010802027423号